Today’s Dietitian
Vol. 18, No. 11, P. 26
Basic Training for RDs About the Pathophysiology
of Type 2 Diabetes and the Role Medications Play
in Managing the Disease
Type 2 diabetes is a complex and progressive disease, so understanding the pathophysiology and the roles of common medications will help dietitians create and present appropriate food-focused treatments to their clients with diabetes.
The following three case studies represent common scenarios that people with type 2 diabetes often present to dietitians.
John, a busy trial lawyer, was diagnosed with type 2 diabetes five years ago. Today is his first appointment with a dietitian. He’s had no diabetes education classes. His primary care provider recently added glyburide, an insulin secretagogue, to his current medication regimen of metformin and sitagliptin (Januvia). John tells you that he doesn’t like the new drug because he thinks it causes dizziness and light-headedness, which he often experiences in the afternoons.
Jean is in the office today for her third appointment with you. When she was diagnosed with type 2 diabetes two months ago, her primary care doctor prescribed metformin. Jean filled the prescription, but hasn’t taken a single pill. She sought you out to help her manage her blood sugar levels without medications. In conversation, she reports that she can’t eat fruit for dessert. She’s tried that a few times and was surprised by her fasting blood sugar numbers the next day. For example, last night her blood sugar was 119 mg/dL about fours hours after eating and right before bed. When she awoke this morning, her blood sugar was 141 mg/dL. She’s certain the fruit has caused her blood sugar to rise during the night.
Louise has had type 2 diabetes for nearly 20 years. She’s been overweight since college and is now at her highest weight with a BMI of 37. She reports usually taking her three diabetes medications, but admits to “not really following her diet.” Her most recent lab report shows an A1c of 8.6%, above her target of less than 7%. Louise has new motivation to tend to her health because last week she found out that her oldest daughter is expecting triplets. She comes to you to help her lose weight. She wants to know how much weight she needs to lose to get rid of her diabetes.
The following review of diabetes pathophysiology and common medications will help dietitians and clients engage in more meaningful MNT sessions.
The Pathophysiology
Diabetes is much more than a blood glucose problem. According to The Art & Science of Diabetes Self-Management Education Desk Reference, published by the American Association of Diabetes Educators (AADE), diabetes is characterized by abnormal metabolism of carbohydrates, proteins, and fats.1 Moreover, having diabetes at least doubles the risk of heart disease.2 However, because blood glucose levels are used to define diabetes and assess control, many people with diabetes focus on these numbers to the exclusion of diet quality and other important health measures. Dietitians can help patients remove their blinders, says Ann Silver, MS, RDN, CDE, CDN, a private practice dietitian for 25 years on the eastern end of Long Island and coauthor of Making Nutrition Your Business: Private Practice and Beyond. Understanding the disease process helps them make sense of their treatment plans, including MNT, she adds.
Disease Process
Years ago, researchers and health care providers described three core defects in type 2 diabetes: failure of the pancreas’ beta cells to produce adequate insulin to maintain blood glucose control and insulin resistance of both the muscle and liver cells. This understanding of the disease led to the widespread use of insulin secretagogues as a primary medical treatment. People with type 2 diabetes took medications that increased the secretion of insulin from the pancreas. Still used today, these drugs are effective at lowering blood glucose and A1c levels, but they don’t treat any underlying causes of the disease.3
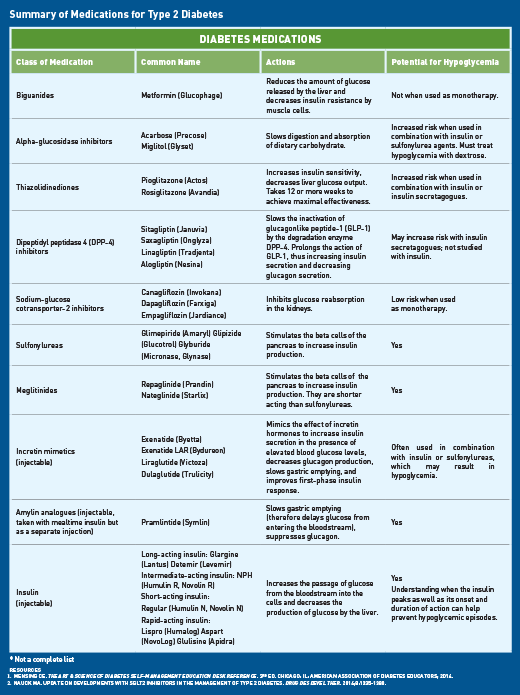
In 2009, Ralph A. DeFronzo, MD, presented a provocative Banting Lecture in which he introduced the Ominous Octet, a total of eight players with a role in the development and progression of type 2 diabetes. This greater understanding of the pathophysiology of type 2 diabetes has led to the development of many medications that target these defects (see table, “Summary of Medications for Type 2 Diabetes” on page 28).
Following are the eight defects in type 2 diabetes as described by DeFronzo in “From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus”3:
Muscle. When the muscle cells are resistant to insulin, they fail to adequately take up glucose from the blood after the ingestion of carbohydrate, resulting in postprandial hyperglycemia.
Liver. The liver is the main player in blood glucose control during fasting. Even in the presence of hyperglycemia and high fasting insulin levels, the liver overproduces glucose. In addition, following a meal, insulin is unable to prevent liver glucose production.
Beta cells of the pancreas. Early in the development of insulin resistance, the pancreatic beta cells secrete higher-than-normal levels of insulin in response to glucose levels. In the first years of this metabolic abnormality, insulin resistance likely goes unnoticed because the higher levels of insulin maintain normoglycemia. As insulin resistance continues, the beta cells lose their ability to secrete high levels of insulin. Thus, blood glucose levels rise to the point of prediabetes and then to type 2 diabetes. It’s the progressive beta cell failure that leads to the progressive nature of type 2 diabetes. It’s estimated that by the time an individual has been diagnosed with type 2 diabetes, 80% of his or her beta cell function has been lost. Though the reason for loss of beta cell function is unclear, one theory is that the cells wear themselves out by oversecreting insulin. Another theory, for which there’s evidence, is that fat deposition in the beta cell leads to both impaired insulin secretion and beta cell failure. Chronic hyperglycemia also impairs beta cell function.
Fat. Fat cells are resistant to insulin and the hormone’s normal effect to inhibit fat breakdown. Thus, the bloodstream receives a steady supply of free fatty acids (FFAs). High levels of FFAs exacerbate insulin resistance in both the muscle and liver cells, increase glucose production in the liver, and further impair insulin secretion from the beta cells. In addition, these dysfunctional fat cells secrete compounds leading to inflammation and atherosclerosis, which raise the risk of heart disease.
Gastrointestinal tract. The simple act of eating elicits the release of incretin hormones from the gut. One of these hormones is glucagonlike peptide-1 (GLP-1), which among healthy people promotes satiety and reduces appetite, enhances glucose-dependent insulin secretion in the beta cells, inhibits postmeal glucagon secretion, reduces glucose output from the liver, and helps regulate gastric emptying. GLP-1 is deficient in people with impaired glucose tolerance and type 2 diabetes, which contribute to hyperglycemia.
Alpha cells of the pancreas. In healthy individuals, glucagon, which is released from the alpha cells, protects against low blood glucose levels during fasting. This hormone is counterregulatory to insulin and stimulates glucose production in the liver. People with type 2 diabetes tend to have high levels of glucagon during fasting; this correlates to their increased rate of glucose production in the liver.
Kidneys. Healthy kidneys filter and reabsorb glucose all day, so no glucose appears in the urine. This guarantees adequate glucose for the brain and other neural tissues. However, among people with type 2 diabetes, this important function of the kidneys becomes a detriment to blood glucose levels. In addition, the diabetic kidney appears to have even greater reabsorptive capacity for glucose.
Brain. DeFronzo postulates that insulin resistance may extend to the brain. This could lead to increased food intake, weight gain, and progression from insulin resistance to type 2 diabetes.
In the February 2016 issue of Diabetes Care, Schwartz and colleagues identified three additional pathways of hyperglycemia in diabetes (not specifically type 2 diabetes): increased levels of systemic inflammation, changes in gut microbiota, and reductions in the hormone amylin.4
Role of Diabetes Medications
With a greater understanding of the pathophysiology of type 2 diabetes has come a flurry of new medications. The majority of these medications work to reverse one or more of the pathogenic abnormalities described above rather than simply acting to lower blood glucose levels. Health care providers frequently refer to an algorithm for the medical management of type 2 diabetes. Preferred resources include an algorithm prepared by the American Association of Clinical Endocrinologists and one created by the American Diabetes Association. Though many clinicians encourage lifestyle changes before the initiation of drug therapy, most people with type 2 diabetes will require medications within one year because of the disease’s progressive nature.1 Many other health care providers prescribe medications at diagnosis to affect the root causes of the disease and gain immediate improvements in A1c levels. Research suggests that beginning metformin, an insulin sensitizer, within three months of diagnosis may slow the demise of pancreatic beta cell function and delay disease progression.1 DeFronzo suggests multiple drugs are needed in combination to correct the multiple pathophysiological defects. With progressive decline of beta cell function, many people with type 2 diabetes eventually will need exogenous insulin.
Intersection of Lifestyle Interventions and Medications
According to Illinois-based dietitian Mary Ann Hodorowicz, MBA, RD, LDN, CDE, CEC, diabetes management is like working on a puzzle: “All the pieces have to fit together to achieve optimal A1c levels, and those pieces change over time.” Knowing how the meal plan, medications, and exercise work together make achieving target blood glucose levels more likely.
The type of diabetes medication an individual takes influences the timing of meals as well as the composition of those meals. A patient who takes a drug with the side effect of hypoglycemia has much less flexibility with meals than does an individual who takes a different type of medication, explains Diane Snyder, RD, CDE, diabetes educator and AADE program coordinator at Sentara Norfolk General Hospital in Norfolk, Virginia. Taking either insulin or a drug that causes the pancreas to secrete more insulin requires patients to eat on a schedule and include adequate carbohydrate to prevent hypoglycemia.
Case Studies Revisited
Consider the case of John, the trial lawyer. He describes classic symptoms of hypoglycemia, a common side effect of glyburide and other insulin secretagogues. With further probing, you find that he often skips or delays lunch, a behavior that’s in conflict with his new medication. Also in conflict is eating a very low-carbohydrate meal such as a mixed green salad with grilled fish or chicken. A patient at risk of plummeting blood glucose levels can eat this meal with fruit, bread, or other carbohydrate-containing foods in proper amounts. However, a patient with a different type of medication regimen likely can enjoy a low-carbohydrate meal without risking low blood glucose.
Jean, on the other hand, needs education regarding the disease process and diet. The hypothesis that eating fruit one day will affect her blood glucose level the next day is unlikely. As described earlier, a primary cause of high fasting blood glucose is elevated glucagon levels and increased liver output of glucose. The metformin prescribed for her targets what many refer to as the leaky liver. Metformin’s primary effect is reducing liver gluconeogenesis. A secondary effect is increasing peripheral insulin sensitivity. Moreover, eating fruit provides Jean with a desired sweetness and nutrients beneficial to her health.
Finally, revisit Louise’s situation. She wants to cure herself of diabetes by losing weight. The likelihood of a great reversal of her diabetes is small because Louise has had diabetes for many years. Because of the progressive nature of type 2 diabetes, Louise has lost significant beta cell function and eventually may require exogenous insulin. Weight loss will provide health benefits but likely will be insufficient to manage blood glucose levels without medications. Because type 2 diabetes is progressive, Silver explains to her clients that they need to do more and more, not less and less, as time goes by. And Hodorowicz points out that since diabetes changes over time, practitioners will need to help clients tweak their care plans.
Diabetes is much more than a disease of elevated blood glucose levels. “Looking at the big picture of type 2 diabetes to see how diet fits into it is an important, nuanced approach to medical nutrition therapy,” says Nancy Black, MS, RD, LDN, CDE, inpatient dietitian at Abington Hospital-Jefferson Health in Abington, Pennsylvania. In addition, she notes, dietitians are in a unique position to share the good news that lifestyle changes are powerful tools to better health, even when medications for diabetes and cardiovascular disease risk factors are necessary.
— Jill Weisenberger, MS, RDN, CDE, FAND, CHWC, is a freelance writer and a nutrition and diabetes consultant to the food industry, including Dow AgroSciences, the maker of Omega-9 Oils, a high-oleic canola oil. She has a private practice in Newport News, Virginia, and is the author of Diabetes Weight Loss — Week by Week.
References
1. Mensing CE. The Art & Science of Diabetes Self-Management Education Desk Reference. 3rd ed. Chicago, IL: American Association of Diabetes Educators; 2014.
2. Detection and treatment of chronic complications. In: Burant CF, ed. Medical Management of Type 2 Diabetes. 7th ed. Alexandria, VA: American Diabetes Association; 2012:113-153.
3. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
4. Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes: rationale and implications of the β-cell-centric classification schema. Diabetes Care. 2016;39(2):179-186.


