Today’s Dietitian
Vol. 21, No. 10, P. 24
Fish and seafood packaging may now boast qualified health claims stating that omega-3s can help reduce risk of hypertension and coronary heart disease and lower blood pressure.
In June, the FDA approved the use of certain qualified health claims on foods and dietary supplements containing the omega-3 fatty acids EPA and DHA relating to their ability to reduce the risk of hypertension and coronary heart disease, as well as lower blood pressure.1,2 The decision was in response to a 2014 petition submitted by the Global Organization for EPA and DHA Omega-3 (GOED).
According to Harry Rice, PhD, GOED’s vice president of regulatory and scientific affairs, who spearheaded the health claim petition on behalf of the organization and its members, “The new qualified health claims provide what many, including GOED, consider to be long overdue acknowledgement from the FDA about the blood pressure–lowering benefits associated with EPA/DHA, the primary long-chain omega-3 fatty acids found in fatty fish. Linking EPA/DHA intake to blood pressure reduction provides consumers a benefit to which they can relate.”
Before the announcement of the new qualified health claims, since 2004 the FDA has allowed the following qualified health claim on certain foods and supplements: “Supportive but not conclusive research shows that consumption of EPA and DHA omega-3 fatty acids may reduce the risk of coronary heart disease.”3
Following are the new qualified health claims the FDA announced, which manufacturers may voluntarily use on labels of seafood and other qualifying foods and supplements1:
1. Consuming EPA and DHA combined may help lower blood pressure in the general population and reduce the risk of hypertension. However, FDA has concluded that the evidence is inconsistent and inconclusive. One serving of [name of the food or dietary supplement] provides [ ] gram(s) of EPA and DHA.
2. Consuming EPA and DHA combined may reduce blood pressure and reduce the risk of hypertension, a risk factor for CHD (coronary heart disease). However, FDA has concluded that the evidence is inconsistent and inconclusive. One serving of [name of the food or dietary supplement] provides [ ] gram(s) of EPA and DHA.
3a. Consuming EPA and DHA combined may reduce the risk of CHD (coronary heart disease) by lowering blood pressure. However, FDA has concluded that the evidence is inconsistent and inconclusive. One serving of [name of the food or dietary supplement] provides [ ] gram(s) of EPA and DHA.
3b. Consuming EPA and DHA combined may reduce the risk of CHD (coronary heart disease) by reducing the risk of hypertension. However, FDA has concluded that the evidence is inconsistent and inconclusive. One serving of [name of the food or dietary supplement] provides [ ] gram(s) of EPA and DHA.
4. Research shows that consuming EPA and DHA combined may be beneficial for moderating blood pressure, a risk factor for CHD (coronary heart disease). However, FDA has concluded that the evidence is inconsistent and inconclusive. One serving of [name of the food or dietary supplement] provides [ ] gram(s) of EPA and DHA.
Dietary supplements and conventional foods bearing any of the above claims must contain at least 0.8 g EPA and DHA (combined total) per serving and meet certain other nutrient content criteria. Importantly, the requirement of 0.8 g per serving isn’t a recommended intake level but rather a level of intake the FDA observed to lower blood pressure in limited studies.
Under general health claim requirements, individual foods can’t bear a health claim if they exceed 13 g total fat, 4 g saturated fat, 60 mg cholesterol, and 480 mg sodium per reference amount customarily consumed (RACC), per labeled serving size, and per 50 g if the RACC is 30 g or less or two tablespoons or less. Furthermore, to bear a health claim, individual foods also must contain, before any nutrient addition, at least 10% DV for vitamin A, vitamin C, iron, calcium, protein (5 g), or dietary fiber per RACC.1,2
Evidence Base for the EPA/DHA Health Claim
The FDA determined that the overall evidence relating to EPA/DHA intake and blood pressure didn’t meet the “significant scientific agreement” standard required for an authorized health claim, but it did meet the “credible evidence” standard for a qualified health claim.2
To assess whether a beneficial link exists between consuming EPA and DHA together and lowering blood pressure, the FDA evaluated 104 intervention studies analyzing the effect of EPA and DHA from conventional foods, dietary supplements, and prescription drugs on blood pressure in both normotensive and hypertensive people. Only 36 of the 104 studies showed a statistically significant benefit, with durations ranging from four weeks to one year, and combined doses of EPA and DHA ranging from 390 mg per day to 15 g per day.
Based on its findings, the FDA concluded there’s “some credible evidence suggesting a relationship between the combined intake of EPA and DHA from conventional foods, dietary supplements, and prescription drugs and blood pressure reduction. However, this evidence is highly inconsistent.”1
Sources of EPA and DHA, Average Intake
EPA and DHA are added to certain supplements and are naturally found or fortified in some conventional foods, including fatty fish (eg, salmon, mackerel, sardines), fish oils, seaweed, and algal oils. Studies have found that food contributes a small amount of DHA and EPA to total daily omega-3 intakes; the National Institutes of Health estimates the amounts at 40 mg in children and teenagers and about 90 mg in adults.3
Dietary supplements containing omega-3s also contribute to total omega-3 intake, with fish oil as one of the most commonly used sources. According to 2012 data, 7.8% of US adults and 1.1% of US children reported taking supplements containing fish oil, omega-3s, and/or DHA or EPA.4,5 According to 2003–2008 National Health and Nutrition Examination Survey (NHANES) data, supplements add about 10 mg to average DHA intakes, and 20 mg to average EPA intakes in adults.6 Data from the FDA and NHANES indicate that the current average intake of EPA and DHA in the United States is only about 77 mg per day from all sources for people aged 4 and older.1
Currently, there’s no recommended intake level in the United States for EPA or DHA. However, to help improve heart health, the 2015–2020 Dietary Guidelines for Americans (DGA) recommend individuals in the general population to consume about 8 oz per week of a variety of seafood, providing about 250 mg per day of EPA and DHA. Importantly, the DGA note that this recommendation is for the total package of nutrients seafood provides, including its EPA and DHA content.7
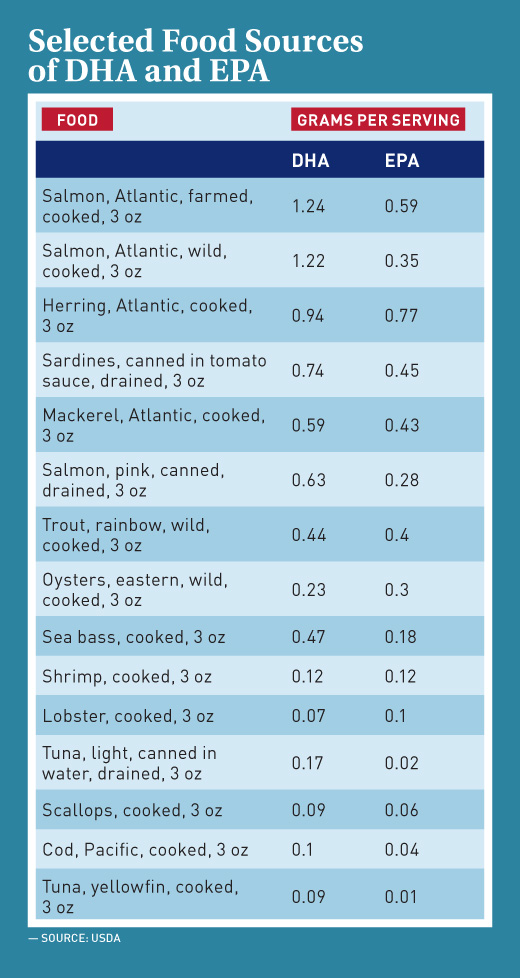
Use of the EPA/DHA Health Claim on Fish
For items sold at seafood counters in supermarkets, nutrition labeling of fresh fish and packaged single-ingredient fish is voluntary unless nutrient content or a health claim is made. Fish and packaged seafood may bear the claim if they meet all the required criteria for its use.
The FDA defines fish as “fresh or saltwater finfish, crustaceans, other forms of aquatic animal life (including, but not limited to, alligator, frog, aquatic turtle, jellyfish, sea cucumber, and sea urchin, and the roe of such animals) other than birds or mammals, and all mollusks, where such animal life is intended for human consumption.” With regard for labeling these products with the EPA/DHA qualified health claims, the FDA considers “products that are essentially all fish” to be those without any added ingredients and with an “insignificant amount” of added fat or carbohydrate. Examples of products considered all fish include raw, boiled, and broiled fish.1
Recommendations for Dietitians
According to Lauren Harris-Pincus, MS, RDN, owner of NutritionStarringYOU.com and author of The Protein-Packed Breakfast Club, “The FDA has simply confirmed what we already know by allowing companies to place this language on their packaging, which should help consumers feel good about getting more omega-3s, while serving as a tool to make these foods more easily identifiable.”
While the new EPA/DHA health claims relate specifically to blood pressure, it’s unlikely consumers will start seeing these claims on food packages due to their length and inability to fit on food labels. However, approval of the claim still represents an opportunity for dietitians to educate their clients on a food-based approach to healthful eating vs a nutrient-specific approach.
According to the American Heart Association’s 2018 Heart Disease and Stroke Statistics, about 103 million US adults have high blood pressure, but almost one-half of them who have been diagnosed aren’t worried about having a heart attack or stroke.8 Furthermore, data from the International Food Information Council Foundation 2019 Food and Health Survey show consumers who reported seeking specific health benefits from foods rank heart health behind digestive health and energy/body weight. Specifically related to omega-3 fatty acids, 70% of respondents view omega-3s as healthful, and 42% of respondents report trying to consume omega-3s.9
Seafood is recommended by the DGA because of its package of nutrients, including but not limited to EPA and DHA. Focusing on seafood as a healthful choice overall instead of specific and limited nutrients can help encourage clients to choose seafood as a protein source.
Clients also will benefit from education related to other lifestyle factors that can help reduce blood pressure and improve heart health, such as exercise and weight loss/maintenance. Furthermore, dietitians can educate clients about food sources that improve heart health overall and address barriers to increasing seafood intake. RDs can talk with clients about realistic ways to incorporate more seafood into their eating patterns, such as choosing canned or frozen options in addition to fresh. The nonprofit Seafood Nutrition Partnership has resources that can help.
Valerie Agyeman, RDN, of Seafood Nutrition Partnership, says, “There are many important health benefits of seafood, including [those related to] heart health the new health claim addresses. In addition to the FDA, the American Heart Association has for many years encouraged people to eat seafood at least twice a week for heart health—and more if you have specific conditions. We’re finalizing a new resource intended for manufacturers, retailers, restaurants, and those at point of purchase, but we think it will be helpful for dietitians as well. It addresses how the new health claim for omega-3s can be utilized and talks about additional claims many species of seafood can make, such as vitamin D, protein, and healthy fats.”
While dietitian Cara Harbstreet, MS, RD, LD, of Street Smart Nutrition, agrees that the claim could be useful, she worries about decision fatigue among shoppers. “Most Americans fall short of the recommended two servings of fish or seafood per week and this claim can direct them toward conventional foods or dietary supplements that may help them close that gap. However, with the abundance of front-of-package labeling and additional health claims that may appear on foods and supplements, dietitians should be aware of the decision fatigue and overwhelm that many of their clients face in the grocery store,” she says.
Harbstreet suggests that “when possible, share food-focused tips and approachable, flavorful recipes. This can be a small step toward building confidence in the kitchen. Ultimately, this may foster a more positive relationship with food because of the focus on the whole food and the eating experience, as opposed to the isolated nutrients mentioned in the health claim.”
Suggestions for Restaurants and Supermarkets
Restaurants and supermarkets covered by the menu labeling regulations can voluntarily use the new EPA/DHA health claim for menu and retail items and items sold at seafood counters, on menus, and in brochures, or by other similar means. For standard menu items that include an EPA/DHA qualified health claim, information on the amount of EPA and DHA must be provided along with the additional written nutrition information required by the menu labeling regulations.10
One of the challenges of using the EPA/DHA qualified health claim on fresh seafood sold in supermarket service cases is that the seafood must contain 0.8 g of EPA and DHA combined per serving, but portions of fresh seafood often don’t have uniform weights and serving sizes.
Another challenge to restaurants and supermarkets when using the EPA/DHA health claim is integrating the different nutrient analysis requirements for both the menu labeling regulation and health claim regulation. Manufacturers of frozen and packaged fish and seafood that would like to use the claim but don’t have room to include it on their labels may have an opportunity to use it in advertising. Although the FDA regulates food labeling, the Federal Trade Commission regulates advertising and marketing practices for food and may allow more latitude when advertising certain food attributes.
Alternatively, manufacturers can use retail programs such as HealthyAisles, Vestcom’s in-store shelf labeling program that specifically proclaims attributes in a food, such as EPA and DHA content. Retailers can use the program to announce the level of DHA and EPA in a product without referencing blood pressure and having to revise their labels.
Ultimately, the new EPA/DHA health claims are unlikely to increase consumer awareness about the link between EPA and DHA intake and blood pressure reduction. In addition, the research on which this claim is based is limited and inconclusive. Instead of recommending clients specifically seek out foods with these health claims to help lower blood pressure, dietitians should counsel them on lifestyle changes overall that help maintain and decrease blood pressure, as well as food-based instead of nutrient-based recommendations for healthful eating.
— Jessica Levings, MS, RD, realtor, is a freelance writer and food industry consultant. She helps consumers Home in on Health with evidence-based resources via her website at BalancedPantry.com. Follow her on Twitter, Facebook, and Instagram @BalancedPantry to learn more.
References
1. Balentine D. RE: Petition for a health claim for eicosapentaenoic acid and docosahexaenoic acid and reduction of blood pressure in the general population (docket no. FDA-2014-Q-1146). US Food and Drug Administration website. https://www.fda.gov/media/128043/download. Published June 19, 2019. Accessed August 15, 2019.
2. US Food and Drug Administration. Label claims for conventional foods and dietary supplements. https://www.fda.gov/food/food-labeling-nutrition/label-claims-conventional-foods-and-dietary-supplements. Updated June 19, 2018. Accessed August 15, 2019.
3. Omega-3 fatty acids: fact sheet for health professionals. National Institutes of Health, Office of Dietary Supplements website. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/. Updated July 9, 2019.
4. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;79:1-16.
5. Black LI, Clarke TC, Barnes PM, Stussman BJ, Nahin RL. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. Natl Health Stat Report. 2015;78:1-19.
6. Papanikolaou Y, Brooks J, Reider C, Fulgoni VL 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003-2008. Nutr J. 2014;13:31.
7. US Department of Health and Human Services. Dietary Guidelines for Americans 2015–2020: Eighth Edition. http://health.gov/dietaryguidelines/2015/guidelines/. Published January 7, 2016. Accessed August 15, 2019.
8. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics — 2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
9. 2019 Food and Health Survey. International Food Information Council Foundation website. https://foodinsight.org/2019-food-and-health-survey/. Accessed August 10, 2019.
10. US Food and Drug Administration; US Department of Health and Human Services. Food labeling; nutrition labeling of standard menu items in restaurants and similar retail food establishments. Final rule. Fed Regist. 2014;79(230):71155-71259.


