Today’s Dietitian
Vol. 17 No. 7 P. 26
Brain and gut hormones impact eating behavior and weight loss efforts.
Many people who have struggled to lose weight and keep it off suspect there’s more to it than simply eating less and resist the notion that failure is simply a matter of not trying hard enough. The fact that two-thirds of adults in the United States are overweight or obese suggest it’s a more complex situation.1
One component of the complexity is the influence of appetite hormones. During the past few decades, researchers have identified numerous hormones that play a role in overall appetite control.2
“In the overweight and obese, resistance to certain satiety hormones can develop, so these people really may be experiencing more hunger physiologically,” says Marjorie Nolan Cohn, MS, RDN, CDN, ACSM-HES, a national spokesperson for the Academy of Nutrition and Dietetics, a clinician in private practice in New York, and author of The Belly Fat Fix. “Clients typically feel better when they hear this. They say, ‘So, my hunger isn’t just in my head or because I don’t have enough willpower or that I just love food.'”
Practitioners who understand how gut and brain hormones impact eating can help clients navigate the pitfalls encountered in weight control attempts.
Leptin: A Satiety Hormone
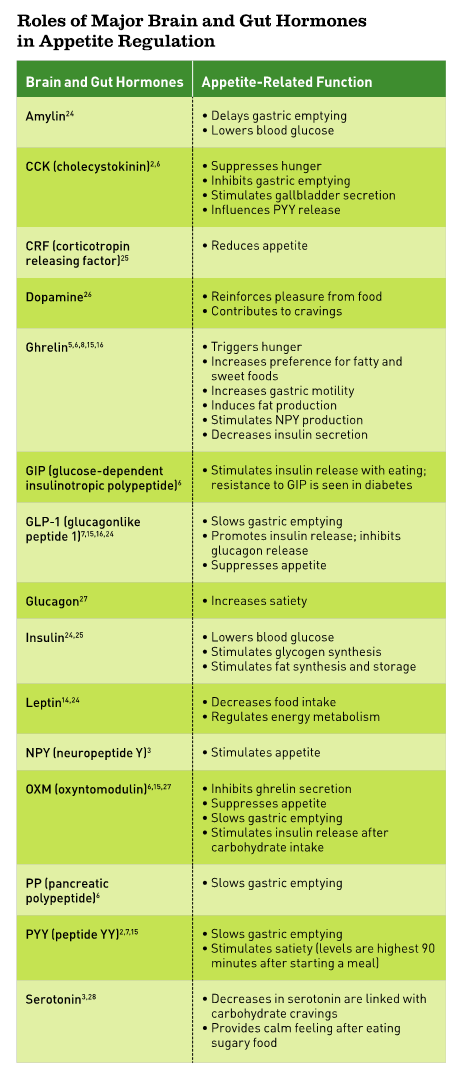
“In the past 20 years, we’ve learned a lot about hunger and satiety hormones,” says Scott Isaacs, MD, a board-certified endocrinologist in Atlanta and author of Beat Overeating Now! “The first one was leptin, which was discovered in 1994.” Since then, many other hormones that impact hunger, appetite, cravings, and weight have been discovered. (See Roles of Major Brain and Gut Hormones in Appetite Regulation sidebar.)
“Leptin is a hormone that’s produced by fat cells, and it works to suppress appetite in the brain,” Isaacs explains. “We used to think a fat cell was an inert storage depot for excess fat, insulating the body. But now we know that fat is an endocrine organ, that is, a gland that produces hormones. Leptin is just one of the hormones produced by fat cells.” The amount of leptin circulating in a person is proportional to the amount of body fat and indicates how much energy stores a person has.3
Nolan Cohn says clients who are morbidly obese often ask why they don’t feel full all the time if leptin is released from fat cells and they have plenty of them due to their excess weight. “I explain that it’s because when someone becomes obese, the body’s cells develop leptin resistance in which leptin receptors don’t bind with the leptin to receive the signal of fullness,” she says.
Not only does hunger increase as a result of this resistance, but according to Isaacs’ book Beat Overeating Now!, metabolism slows, too.4
Inflammation is a big component of this phenomenon, Isaacs says. “Leptin resistance (as well as insulin resistance) is caused by fat cells, especially in visceral or belly fat, producing large numbers of inflammatory chemicals or cytokines, which block the effects of leptin,” Isaacs explains. He adds that eating healthful foods, including ones rich in anti-inflammatory antioxidants and omega-3 fats, can improve leptin resistance. Interestingly, a study by Jönsson and colleagues published in the January 2015 issue of BMC Biochemistry suggests that digested wheat gluten may inhibit binding of leptin to the leptin receptor, but further research is needed to verify that finding.
Ghrelin: The Hunger Hormone
“Ghrelin is the primary and most powerful hunger-stimulating hormone,” Nolan Cohn says. “It’s secreted mainly from the stomach lining and travels through the blood to your brain, signaling that it’s time to eat.” Ghrelin was discovered in 1999, and has other functions beyond hunger stimulation that are still being investigated.5
“Ghrelin works on a cycle, rising before meals and dropping after meals. This happens naturally about every four hours,” Nolan Cohn adds.
Eating at regular intervals—breakfast, lunch, afternoon snack, and dinner—is in sync with this cycle, but after overnight fasting ghrelin levels are increased. Ghrelin levels rise approximately two-fold immediately before a meal, and then decrease to their lowest levels about one hour after a meal.6
“I tell clients that even if dinner is an hour away, if they’re really hungry now, they need to eat something, because ghrelin is a short-acting hormone, meaning that it works minute by minute, hour by hour,” Nolan Cohn says. “There isn’t any sort of cap to ghrelin—it literally builds and builds and builds until we eat.” Moreover, it isn’t affected by what you ate yesterday.5
Although one might think that ghrelin levels are higher in an obese person, thus driving more hunger, the opposite is true. Human studies have found that ghrelin levels actually are lower in the obese, but they’re more sensitive to its appetite-stimulating effects.7 However, in people with a genetic disorder leading to obesity, Prader-Willi syndrome, circulating levels of ghrelin are very high, thus driving excessive hunger and extreme overeating.5,8
Dopamine: The Reward Hormone
Interconnected with satiety hormones are neurotransmitter brain hormones, including dopamine. Dopamine directly activates reward and pleasure centers in the brain, which can affect both mood and food intake.9 Obese individuals often have a blunted dopamine pathway because of chronic exposure to highly palatable foods.10 “This blunted response has been suggested to contribute to increased reward-seeking behavior, including overeating,” says Heather Leidy, PhD, an assistant professor of nutrition and exercise physiology at the University of Missouri in Columbia.
“Eating increases dopamine, and the majority of studies have shown increased dopamine with intake of high-fat foods,” Leidy says.
Dopamine levels also rise with sugar intake.11 However, both high fat and high sugar foods can lead to increased appetite, overeating, and weight gain over the long term.10
In the August 2014 issue of Nutrition Journal, Leidy and colleagues describe the results of their randomized, crossover pilot study comparing satiety effects from high-protein breakfasts (containing 35 g of high-quality animal protein) vs normal-protein breakfasts (13 g) or breakfast skipping in overweight and obese late-adolescent girls. The high-protein breakfast was best at reducing postmeal cravings and increasing dopamine levels.10 Leidy says this study was the first to show that dopamine increases when you eat protein. “Protein contains amino acids, several of which are the building blocks of dopamine. Thus, increasing protein consumption has been suggested to also increase dopamine production,” Leidy says.
In addition, getting enough of one amino acid, tyrosine, prevents a bottleneck in dopamine synthesis, since it’s required in the rate-limiting step.10 Top tyrosine sources include meat, poultry, eggs, fish, cheese, soybeans, and peanuts.12 Reaching 35 g of protein takes a bit of planning, though. “A breakfast containing eggs (and egg whites), lean meats, and dairy (particularly Greek yogurt) can give you 35 g of high-quality protein,” Leidy says.
Although Leidy’s study was done with obese participants, she says there also are data showing improved satiety and reduced appetite with the consumption of a high-protein breakfast in normal-weight individuals.
The Interplay of Genetics
Genetics also can affect how dopamine and other brain hormones work.
“If you have the A1 variant (rs1800497) of the DRD2 dopamine receptor gene, which controls synthesis of dopamine D2 receptors, you’ll have 30% to 40% fewer dopamine receptors, which can contribute to addiction risk, such as to food, alcohol, or drugs,” says Kenneth Blum, PhD, a neuroscientist who codiscovered this DRD2 addiction gene variant in 1990 and is a professor in the department of psychiatry and the McKnight Brain Institute at the University of Florida.
The DRD2 variation is carried by about one-third of the US population, which is about 100 million people.9 And, it’s well-established that there’s an increased prevalence of the DRD2 A1 allele in obese individuals.13
“When individuals have the A1 allele of the D2 dopamine receptor gene, the brain response to food (and other forms of pleasure) is blunted, so they don’t perceive that they’ve been satiated and overeat and gain weight,” Blum says. The gene variant can contribute to carbohydrate bingeing, including sugar, which stimulates the brain’s production and utilization of dopamine and therefore can improve mood.11 “The DRD2 A1 gene variant also increases the number of fat cells in the body,” he says.
Blum adds that although the dopamine D2 receptor mutation likely plays a significant role in obesity, it’s just one among many possible genetic variants. “People can have genetic variants all across the brain-reward cascade, such as in serotonin or GABA [gamma-aminobutyric acid], too,” Blum says. “When we look at controlling obesity, we’re looking at more than 600 genes. Interestingly, all of them impinge on dopamine release.”
Another gene that can increase fat cell production and influence appetite is the FTO (fat-mass and obesity-associated) gene, Blum says. In a Swedish study published in the November 2014 issue of Diabetes, Benedict and colleagues found that elderly men and women who had one variant for the common FTO C allele (rs17817449) had significantly increased circulating levels of hunger-promoting ghrelin and decreased levels of satiety-promoting leptin after an overnight fast compared with those without the variant in that pair of alleles. This sets up those with the variant to have increased hunger.14 The same study found individuals who possessed the variant in both alleles had an even greater increase in ghrelin and decrease in leptin levels than those with just one variant allele.
Fortunately, genetics isn’t always destiny. Epigenetic effects—the effects of the environment on DNA, such as home life, family support, and stress—can overcome some genetic traits.9 Just because someone carries a genetic variant doesn’t mean it’s being expressed. A complex interplay of genetic mutations and environmental conditions is what ultimately impacts obesity risk.7
“Over the last 10 years, we’ve found that a person’s environment can have a profound effect on the expression of genes, sometimes affecting multiple generations,” Blum says. “For example, if someone exercises, it can turn off the expression of the FTO gene, so you get less fat cells.”
Bariatric Surgery
Bariatric surgery can affect appetite-regulating hormones as well as dopamine receptors. Two satiety hormones, PYY (peptide YY) and GLP-1 (glucagonlike peptide 1), usually are increased after Roux-en-Y gastric bypass (RYGB) surgery, and ghrelin levels are typically lower, which have been shown to help reduce food intake.15,16 In addition, after RYGB, a decrease in dopamine D2 receptor availability has been found, which Blum suggests may have unintended consequences in some individuals.
“A certain percentage of people who go in for bariatric surgery carry genes that reduce dopamine levels, such as the DRD2 A1 allele. These people may really be addicted to sugar or overeating, just as people are addicted to alcohol or gambling, since all of them cause dopamine release,” Blum says. Blum has been involved in research showing that in animal models of addiction, withdrawal from sugar causes imbalances in neurotransmitters, including dopamine, in a manner similar to opiate withdrawal.13
“So, even though a person’s food addiction problem may go away after bariatric surgery, sometimes they still don’t feel right,” Blum says. “They look for something else to bring their dopamine back up since they’re not getting as much from food anymore. Some may start to drink alcohol, gamble, do drugs, or pick up some other behavioral addiction. The root cause may be that they just don’t have enough dopamine. We call this reward-deficiency syndrome.”
To help identify such individuals, Blum led the development of a genetic test called the Genetic Addiction Risk Score, which measures genes all across the reward systems.17 He says the test will become available this fall through Dominion Diagnostics (dominiondiagnostics.com). “Testing doesn’t solve the addiction tendency, but at least people will know this risk before going in for bariatric surgery,” Blum says. “At a minimum, at least 20% of people have this transfer of addiction, but it’s probably more like 40% to 50%.”
Appetite Hormone Medications
Medications to regulate appetite hormones are in the experimental phase, Isaacs says. Only the drug Saxenda (liraglutide) is FDA approved and currently available, he says. Saxenda is a synthetic version of a feel-full hormone (GLP-1) that’s self-injected daily and slows gastric emptying. It’s the same medication as the diabetes drug Victoza, so Saxenda shouldn’t be used with Victoza.18
Many attempts at using medications to impact appetite hormone regulation either haven’t worked or produced unacceptable side effects. Pharmaceuticals that acted as ghrelin antagonists were developed as antiobesity drugs, but they failed to reduce food intake.5 Similarly, giving someone extra PYY, which promotes satiety, can cause several gastrointestinal side effects, including nausea.2 Moreover, oral doses of PYY aren’t absorbed well, and injections of PYY aren’t as effective as what the body secretes naturally.2 Nonetheless, pharmaceutical companies are still working to develop successful appetite hormone-based treatments for obesity.15
Blum adds that some pharmaceutical companies have tried to develop drugs to treat food (and drug) addiction by blocking dopamine, but those efforts have backfired. “Blocking dopamine over the long term can result in autoimmune changes, depression, and suicide,” he says. “Similarly, if you give someone an agonist that stimulates the dopamine D2 receptors, the brain thinks the body has too much dopamine, so it down-regulates or reduces the number of dopamine receptors.”
In Blum’s view, what’s needed is a substance that would regulate dopamine. He’s been researching the use of a nonaddictive amino acid dietary supplement called KB220Z to normalize brain dopamine in those with low D2 receptors due to their genetics and has seen some encouraging results. He says KB220Z is sold in different forms as a dietary supplement under several brand names.
Managing Appetite Hormones
Research into the role of hormones in hunger, satiety, and weight control continues, but what can dietitians do to help clients and patients? The following 10 tips can help stabilize hormone levels that affect hunger:
1. Eat on a schedule. “This helps prevent wide swings in appetite hormones, so you don’t get overly hungry, and it reduces the likelihood you’ll overeat, especially in the evening, which is when 90% of people are overeating,” Isaacs says.
2. Eat a high-protein breakfast. People who say they aren’t hungry for breakfast often will notice a shift to experiencing morning hunger relatively soon after they adopt a breakfast habit. A rise in morning ghrelin is part of that shift, and eating breakfast helps stabilize hunger for the entire day, Nolan Cohn says. Protein reduces ghrelin levels best, and generally increases leptin activity, too.6,19,20
3. Eat a mix of macronutrients at meals and snacks. Protein is best at stimulating release of many satiety hormones, but carbohydrates and fat are more effective for stimulating certain satiety hormones, such as GIP (glucose-dependent insulinotropic polypeptide) and GLP-1, respectively.2,6,19
4. Consume omega-3 fats. Omega-3 fats, such as those from salmon and DHA-fortified dairy products, can increase the number of dopamine receptors and dopamine levels, Blum says. Omega-3 fats also are anti-inflammatory and may help improve insulin and leptin sensitivity.21
5. Eat ghrelin-suppressing foods at each meal. Topping the list are high-quality animal proteins, such as skinless poultry, lean beef, fish, eggs, and fat-free Greek yogurt, Nolan Cohn says. She also emphasizes unprocessed carbohydrates high in resistant (nondigestible) starch, such as lentils, oats, and sweet potatoes, which may help boost satiety hormones, including PYY and GLP-1.22
6. Plan meals with low energy density (fewer calories per bite). Meals need to look visually filling in terms of volume, Nolan Cohn says. Research from Penn State University has linked low energy-dense diets with lower circulating levels of ghrelin and a trend toward higher circulating concentrations of PYY.23
7. Seek pleasure from other activities. The classic approach of finding an alternate activity to get your mind off a craving has benefits beyond distraction. Activities such as listening to music and doing yoga also increase dopamine levels, thus providing pleasure, Blum says.
8. Get adequate sleep (generally seven to eight hours). “When people get just two hours less sleep than what their body needs, their ghrelin levels will be higher the next day,” Nolan Cohn says. Insufficient sleep also can negatively impact leptin and insulin levels.19
9. Commit to regular exercise. Physical activity not only burns calories but also can increase levels of certain satiety hormones, such as PYY and CRF (corticotropin releasing factor), and reduce leptin resistance.1-3
10. Enlist support of mental health experts for bariatric surgery patients. This can help in identifying signs of transfer of addiction so they can promptly obtain assistance.13
— Marsha McCulloch, MS, RD, LD, is a nutrition writer and consultant in South Dakota.
References
1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491-497.
2. Cooper JA. Factors affecting circulating levels of peptide YY in humans: a comprehensive review. Nutr Res Rev. 2014;27(1):186-197.
3. Friedman JM, Mantzoros CS. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism. 2015;64(1):1-4.
4. Isaacs S. Beat Overeating Now!: Take Control Of Your Hunger Hormones To Lose Weight Fast. Beverly, MA: Far Winds Press; 2012:66-68, 147.
5. Kirchner H, Heppner KM, Tschöp MH. The role of ghrelin in the control of energy balance. In: Appetite Control. Berlin, Heidelberg: Springer; 2012:161-184.
6. Adamska E, Ostrowska L, Górska M, Krętowski A. The role of gastrointestinal hormones in the pathogenesis of obesity and type 2 diabetes. Prz Gastroenterol. 2014;9(2):69-76.
7. Skelton JA, DeMattia L, Miller L, Olivier M. Obesity and its therapy: from genes to community action. Pediatr Clin North Am. 2006;53(4):777-794.
8. Solomou S, Korbonits M. The role of ghrelin in weight-regulation disorders: implications in clinical practice. Hormones. 2014;13(4):458-475.
9. Blum K. The addictive brain: all roads lead to dopamine. Collier’s website. http://colliersmagazine.com/article/addictive-brain-all-roads-lead-dopamine. Updated April 2012. Accessed April 24, 2015.
10. Hoertel HA, Will MJ, Leidy HJ. A randomized crossover, pilot study examining the effects of a normal protein vs high protein breakfast on food cravings and reward signals in overweight/obese “breakfast skipping,” late-adolescent girls. Nutr J. 2014;13:80.
11. Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014;5:919.
12. United States Department of Agriculture. National Nutrient Database for Standard Reference, Release 27. http://ndb.nal.usda.gov/ndb/nutrients/index. August 2014.
13. Blum K, Bailey J, Gonzalez AM, et al. Neuro-genetics of reward deficiency syndrome (RDS) as the root cause of “addiction transfer”: a new phenomenon common after bariatric surgery. J Genet Syndr Gene Ther. 2011;2012(1):S2-001.
14. Benedict C, Axelsson T, Söderberg S, et al. Fat mass and obesity-associated gene (FTO) is linked to higher plasma levels of the hunger hormone ghrelin and lower serum levels of the satiety hormone leptin in older adults. Diabetes. 2014;63(11):3955-3959.
15. De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6(1):10-20.
16. Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110(4):571-584.
17. Blum K, Oscar-Berman M, Demetrovics Z, Barh D, Gold MS. Genetic addiction risk score (GARS): molecular neurogenetic evidence for predisposition to reward deficiency syndrome (RDS). Mol Neurobiol. 2014;50(3):765-796.
18. Saxenda. Saxenda website. https://www.saxendapro.com/. Accessed April 27, 2015.
19. Schwarz NA, Rigby BR, La Bounty P, Shelmadine B, Bowden RG. A review of weight control strategies and their effects on the regulation of hormonal balance. J Nutr Metab. 2011;237932.
20. Izadi V, Saraf-Bank S, Azadbakht L. Dietary intakes and leptin concentrations. ARYA Atheroscler. 2014;10(5):266-272.
21. Abete I, Parra D, Crujeiras AB, Goyenechea E, Martinez JA. Specific insulin sensitivity and leptin responses to a nutritional treatment of obesity via a combination of energy restriction and fatty fish intake. J Hum Nutr Diet. 2008;21(6):591-600.
22. Keenan MJ, Martin RJ, Raggio AM, et al. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: a microarray study. J Nutrigenet Nutrigenomics. 2012;5(1):26-44.
23. Hill BR, Rolls BJ, Roe LS, De Souza MJ, Williams NI. Ghrelin and peptide YY increase with weight loss during a 12-month intervention to reduce dietary energy density in obese women. Peptides. 2013;49:138-144.
24. Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57(5):359-372.
25. Diz-Chaves Y. Ghrelin, appetite regulation, and food reward: interaction with chronic stress. Int J Pept. 2011;898450.
26. de Weijer BA, van de Giessen E, Janssen I, et al. Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia. 2014;57(5):1078-1080.
27. Bewick GA. Bowels control brain: gut hormones and obesity. Biochem Med (Zagreb). 2012;22(3):283-297.
28. Lysen LK, Israel DA. Nutrition in weight management. In: Mahan LK, Raymond JL, Escott-Stump S. Krause’s Food and the Nutrition Care Process. 13th ed. St. Louis, MO: Elsevier; 2011:466-467.


