 June/July 2025 Issue
June/July 2025 Issue
CPE Monthly: Managing Hyperhomocysteinemia
By Mary Franz, MS, RDN
Today’s Dietitian
Vol. 27 No. 6 P. 36
Take this course and earn 2 CEUs on our Continuing Education Learning Library
Homocysteine is a sulfur-containing nonessential amino acid that is formed in the body during the metabolism of dietary methionine to cysteine. Homocysteine is nonproteinogenic, meaning that it is not found in proteins and is not incorporated into the genetic code. Additionally, homocysteine is not found in foods and is synthesized only from dietary methionine. Methionine is an essential amino acid obtained primarily from dietary animal protein, such as meat, poultry, fish, eggs, and dairy products.1
An elevated level of homocysteine in the body is known as hyperhomocysteinemia, a condition that is linked to the development of many chronic disorders, including CVD, diabetes, renal disease, and neurocognitive disorders. Individuals with hyperhomocysteinemia may not be diagnosed due to the disorder’s vague symptoms. Nutrition professionals, therefore, need to be aware of the importance of proper diagnostic tests and treatment regimens for this disorder.
Diagnosis and Symptoms of Hyperhomocysteinemia
Hyperhomocysteinemia is diagnosed by measuring blood levels of homocysteine. Normal plasma levels of homocysteine range from 5 to 15 umol/liter. Plasma levels higher than this range are classified as mild/moderate, intermediate, or severe hyperhomocysteinemia2:
• mild/moderate: 15 to 30 μmol/L;
• intermediate: 30 to 100 μmol/L; and
• severe: >100 μmol/L.
Hyperhomocysteinemia is often asymptomatic; however, mild to intermediate levels of the disorder may present with nonspecific symptoms, such as dizziness; muscle weakness; fatigue; tingling in hands, feet, legs, or arms; and weight loss. More serious symptoms occur with severely elevated blood levels of homocysteine and include osteoporosis, hip fractures, visual disturbances, kidney disease, hypothyroidism, cognitive decline, and other neurological problems.3
Abnormal blood levels of folate, vitamin B6, and vitamin B12 are also associated with hyperhomocysteinemia. Because serum and plasma B12 testing may produce inaccurate results, methylmalonic acid (MMA) testing is the preferred method for determining B12 status. Methylmalonic acid is a byproduct of protein digestion that requires vitamin B12 for complete breakdown. Elevated blood levels of MMA (greater than 0.37 umol/L) may indicate vitamin B12 deficiency. The following values are plasma threshold levels that indicate deficiencies of these vitamins4-6:
• folate: < 3 ng/mL;
• vitamin B6: < 20 nmol/L; and
• vitamin B12: < 200 pg/mL.
Prevalence, Causes, and Risk Factors for Hyperhomocysteinemia
The estimated prevalence of hyperhomocysteinemia in the general population is 5% to 7%.3 Hyperhomocysteinemia is higher among men (45.4%), compared with women (28.5%). The risk of developing the disorder increases with age and is highest among individuals over 80 years old. Black and Asian individuals have a higher risk of developing hyperhomocysteinemia compared with individuals of other ethnicities. Other important risk factors for hyperhomocysteinemia include obesity and cigarette smoking.1,3,7
Congenital defects in the enzymes that regulate homocysteine metabolism are a primary cause of hyperhomocysteinemia. These defects are inborn errors of metabolism that affect all stages of homocysteine metabolism.2
Dietary folate, vitamin B6, and vitamin B12 play a significant role in regulating plasma levels of homocysteine. Vegan and restricted diets may be deficient in one or more of these vitamins and possibly increase the risk of hyperhomocysteinemia. Illness and malabsorption can also lead to deficiencies of these vitamins.1,2
Many medications, including anticonvulsants, cholesterol-lowering drugs, metformin, methotrexate, and thiazide diuretics, can also increase plasma homocysteine levels.1
Diet plays a key role in regulating plasma homocysteine. Excessive intakes of animal protein, alcohol, added sugars, and processed foods have been tied to increased rates of hyperhomocysteinemia. A study conducted among an estimated 6,000 adult men and women examined the effects of different foods on plasma homocysteine levels. Intakes of fruit, vegetables, whole-grain bread, and nonprocessed meats were associated with lower plasma homocysteine levels, whereas consumption of sweets, cake, and processed meats were significantly related to elevated homocysteine levels. Foods rich in B vitamins and low in sugar and fat were also inversely related to blood homocysteine concentrations.8
Biosynthesis and Regulation of Homocysteine
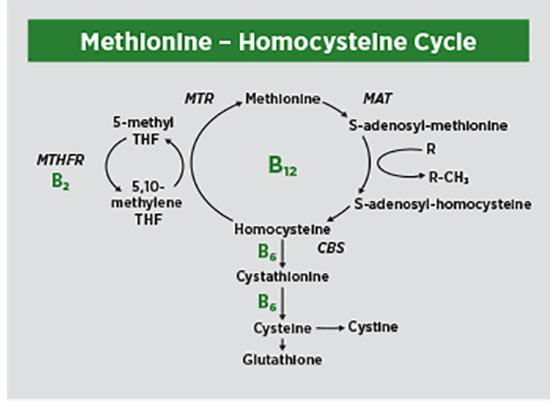
The biosynthesis and regulation of homocysteine in the body is a complex process involving several distinct metabolic pathways. Each of these pathways is controlled by an enzyme(s) essential for homocysteine regulation. The biosynthesis of homocysteine from methionine, referred to as the “methionine cycle,” occurs in three steps1,2,9:
1. Dietary methionine receives an adenosyl group from ATP to form S-adenosyl-L-methionine (SAM). The enzyme methionine adenosyltransferase (MAT) controls this step in the reaction.
2. The SAM molecule is a universal methyl donor that is active in the methylation of many acceptor compounds, such as DNA, RNA, proteins, creatine, and phospholipid membranes. The SAM molecule donates a methyl group to one of these compounds, usually a protein, through a pathway called demethylation. This process converts SAM to S-adenosyl homocysteine (SAH).
3. Homocysteine is formed when adenosine is removed from the SAH molecule. This process is reversible. Homocysteine can be converted back to methionine by the enzyme methionine synthase (MTR).
Homocysteine then undergoes one of two processes in the body:
1. Transsulfuration: This process occurs in the liver and kidneys and converts homocysteine to the amino acid cysteine. The process is irreversible and is controlled by the activity of two enzymes: cystathionine beta-synthase (CBS) and cystathionine gamma-lyase, both of which require the active form of vitamin B6, pyridoxal 5’-phosphate, to function. The cysteine formed during the process is then used to produce glutathione or to synthesize proteins. A deficiency of vitamin B6 can suppress transsulfuration, and lead to increased free radical production and oxidative stress.7,9
2. Remethylation: Homocysteine may be remethylated back to methionine by accepting a methyl group from the biologically active form of folate, 5-methyltetrahydrofolate (5-MTHF). In the second step, the enzyme MTR completes the remethylation of homocysteine to methionine. MTR requires the active form of vitamin B12, methylcobalamin, for functionality. The remethylation pathway can be disrupted or blocked by deficiencies of folate and vitamin B12.7,9
The body can also use betaine as a methyl donor in the remethylation pathway. Betaine is a modified nonessential amino acid obtained from dietary sources (eg, beets, spinach, wheat germ, whole grains) and is also formed endogenously in the liver and kidneys from dietary choline, which is found in meat, poultry, eggs, fish, and dairy foods.10
The processes of transsulfuration and remethylation are synchronized to tightly regulate homocysteine levels. Both pathways are dependent on the cellular levels of the SAM molecule, which is the most important regulator of homocysteine metabolism. The high plasma levels of SAM that occur after a protein-rich meal inhibit the enzyme methylenetetrahydrofolate reductase (MTHFR), activate CBS, and cause homocysteine to be converted to cysteine. Alternatively, when dietary protein intake is low and blood levels of SAM fall, homocysteine undergoes remethylation to form methionine. Defects in either transsulfuration and remethylation will lead to disruptions in the metabolism of homocysteine and elevated plasma levels of homocysteine.9
Enzyme Deficiencies and Hyperhomocysteinemia
The transsulfuration and remethylation pathways rely on the activity of enzymes to function properly. Defects in enzymes that metabolize homocysteine are a primary risk factor for hyperhomocysteinemia.7,9 A summary of the key enzymes responsible for homocysteine metabolism follows.
CBS
CBS is the first enzyme in the transsulfuration cycle. CBS converts homocysteine to cysteine. A deficiency of CBS is caused by a mutation of the CBS gene, and results in homocystinuria, a rare inborn error of metabolism in which plasma and urinary homocysteine levels are elevated above normal ranges. Symptoms of homocystinuria may be mild, intermediate, or severe, and include ocular disorders, vascular abnormalities, skeletal deformations, and intellectual disabilities. In the United States, newborns are typically screened for CBS deficiency by measuring blood levels of methionine; however, the sensitivity of this screening test is low, and the condition is often undiagnosed. The estimated prevalence of homocystinuria in the United States is 1 in 100,000 to 200,000, but the actual prevalence may be much higher, due to the inaccuracy of the screening test. Genetic testing for abnormalities in the CBS gene is more accurate, but not routinely performed.11
Newborns diagnosed with CBS deficiency are treated with methionine-free formulas. These formulas contain all the nonessential and essential amino acids except methionine and are supplemented with betaine, B vitamins, and cystine, a precursor to the antioxidant glutathione. Low-protein diets and supplementation with betaine, choline, folic acid, and vitamins B6 and B12 are standard therapies for older infants and children with CBS deficiency.12
MTHFR
The MTHFR enzyme controls the conversion of inactive folate (5,10-methylenetetrahydrofolate) to active folate, 5-MTHF. A deficiency of the MTHFR enzyme is caused by mutations of the MTHFR gene (at least 40 mutations have been identified). Individuals with limited or absent activity of the MTHFR enzyme cannot metabolize folate as efficiently, a necessary step for the remethylation of homocysteine. The prevalence of MTHFR enzyme deficiency is estimated to be about 10% to 15% of the general population. The condition is diagnosed by genetic testing or by measuring the plasma homocysteine level. The typical treatment is supplementation with oral betaine, folic acid, vitamin B6, and vitamin B12.7,9
MTR
The MTR enzyme catalyzes the regeneration of methionine from homocysteine. The activity of MTR is regulated by the MTRR gene. Mutations of this gene cause deficiencies of MTR, making the body unable to convert homocysteine to methionine. Symptoms of MTR deficiency include seizures, encephalopathy, macrocytic anemia, hypotonia, developmental delay, and psychiatric symptoms or neurologic changes. The prevalence of MTR deficiency is unknown. The disorder is diagnosed by genetic testing or by blood tests that measure methionine or homocysteine levels. The accepted treatment is daily intramuscular injections of vitamin B12 and supplemental oral betaine.9,13
MAT
The MAT enzyme controls the transfer of an adenosyl group from ATP to methionine to form SAM, the first step in the methionine cycle. A deficiency of MAT is caused by mutations in the MAT1A gene and results in abnormal myelination of nerve cells and developmental delay. Treatment of MAT deficiency includes supplementation with betaine, folic acid, vitamin B6, and vitamin B12, and a methionine-restricted diet.14
Folate, Vitamin B6, and Vitamin B12 Deficiencies
Adequate levels of folate, vitamin B6, and vitamin B12 are needed to properly metabolize homocysteine. Deficiencies of one or more of these vitamins can interfere with the body’s regulation of homocysteine and lead to hyperhomocysteinemia. Folate, vitamin B6, and vitamin B12 deficiencies are due to poor dietary intakes, illness, genetic defects that result in impaired absorption or transport, or a combination of these factors.9
Although clinical deficiencies of these vitamins are uncommon in the United States and are usually limited to individuals with genetic mutations, low or marginal deficiencies have been reported, particularly among nonwhite and elderly populations, and women of childbearing age. Current estimates of subclinical folate, vitamin B6, and vitamin B12 status within the overall population vary from 5% to 25%, and are due to variation in the bioavailability of food sources, differences in dietary assessment methods, and potential limitations of diagnostic tests.15-17
Folic Acid and Folate
Although the terms folic acid and folate are used interchangeably, folic acid refers to the oxidized synthetic form of the vitamin, whereas folate is the reduced form found naturally in foods and sometimes considered an umbrella term capturing folic acid as well. The main dietary sources of folate are leafy green and cruciferous vegetables, citrus fruit, and legumes, and breakfast cereals fortified with synthetic folic acid.
Both folic acid and folate from food must be converted to the active form 5-MTHF to become biologically active. After ingestion, folate from food is hydrolyzed in the gut, where it is converted first to tetrahydrofolate by the enzyme dihydrofolate reductase and then to the active form 5-MTHF, by the enzyme MTHFR. Metabolism of synthetic folic acid occurs in the liver by the same processes. Erythrocyte folate concentrations provide an accurate measure of long-term folate intakes; a concentration above 140 ng/mL indicates adequate folate status.4
The enzyme dihydrofolate reductase is responsible for the conversion of dietary folate to tetrahydrofolate and is controlled by the DHFR gene. A rare mutation of this gene causes disturbed folate metabolism, which may result in hyperhomocysteinemia, as well as neurologic disorders and developmental delays.18
Vitamin B6
Pyridoxine, pyridoxamine, and pyridoxal are the forms of vitamin B6 that are found in foods. Dietary sources of vitamin B6 include chickpeas, beef, fish, liver, poultry, starchy vegetables, wheat germ, and fortified cereals. After absorption in the gut, dietary vitamin B6 is converted in the liver to its biologically active forms, pyridoxal 5’-phosphate and pyridoxamine-5’-phosphate, which are coenzymes in homocysteine metabolism.5 The endogenous conversion of dietary vitamin B6 to its active forms is regulated by the enzyme pyridoxamine-5’-phosphate oxidase. A defect in the PNPO gene that controls this enzyme leads to vitamin B6 deficiency and blocks the transsulfuration pathway.9,19
Vitamin B12
Vitamin B12 is essential for the remethylation of homocysteine to methionine. A key enzyme in this pathway, MTR, requires the presence of B12, which functions as a cofactor in the pathway.9
The active form of vitamin B12, methylcobalamin, is found in meat, fish, poultry, and dairy products, as well as fortified breakfast cereals. Because plant foods do not contain vitamin B12, vegans and vegetarians are at risk of developing deficiency and need to incorporate other sources of vitamin B12 into their diets, such as supplements, fortified breakfast cereals and plant milks, nutritional yeast, and fermented soybeans (tempeh).6
In addition to inadequate dietary intakes, vitamin B12 deficiency is caused by diminished or absent production in the gut of hydrochloric acid and intrinsic factor, both of which promote the absorption, transport, and uptake of vitamin B12 by cells. Globally, pernicious anemia, a condition in which the parietal cells of the stomach fail to produce intrinsic factor, is the most common cause of vitamin B12 deficiency. Individuals who don’t produce intrinsic factor need 1,000 to 2,000 mcg of supplemental vitamin B12 daily. Oral and intramuscular B12 supplementation appear to have comparable effects on normalizing serum B12 levels.6
Mechanisms of Disease Development and Potential Treatment of Hyperhomocysteinemia
The harmful effects of homocysteine are believed to be due to the sulfhydryl group in its structure. Sulfhydryl groups are highly reactive molecules easily oxidized to form hydrogen peroxide and reactive species. These toxic substances trigger the release of proinflammatory cytokines that contribute to oxidation and the onset of chronic disease.20
Hyperhomocysteinemia also stimulates the production of toxic substances within cells and tissues. A compound called homocysteine thiolactone forms when homocysteine incorrectly binds to lysine residues of cellular proteins, leading to damage in the cell structure. Homocysteine may also react with nitric oxide in the body to form S-nitrosohomocysteine, a compound that causes endothelial damage and reduces the vasodilatory function of blood vessels.2,21
The primary goal in treating hyperhomocysteinemia is to reduce elevated plasma homocysteine levels in order to minimize the risk of neurological and vascular complications. The standard treatment for hyperhomocysteinemia is supplementation with folate, vitamin B6, and vitamin B12. Individuals with inborn errors of metabolism that affect homocysteine metabolism may require methionine-restricted diets and supplemental betaine and amino acids.21
CVD
The American Heart Association recognizes hyperhomocysteinemia as an independent risk factor for CVD, atherosclerosis, stroke, thromboembolism, and myocardial infarction.7 Some research has demonstrated correlations between plasma homocysteine levels and the severity of atherosclerotic disease, as well as associations between hyperhomocysteinemia and increased risk of cardiovascular death.
In 2022, Wang and colleagues published findings from a meta-analysis that investigated plasma homocysteine levels and the risk of coronary heart disease. The studies were conducted in the United States, China, and Europe among 10,103 adult participants. For every 5 umol/L increase in plasma homocysteine, there was a 20% increase in the risk of coronary heart disease.22 These findings were confirmed by another review of 35 studies conducted in 14 countries among 27,448 adults and children aged 5 to 60 years. The risk of atherosclerotic disease was strongly associated with elevated homocysteine levels. Homocysteine levels were also correlated with obesity, smoking, defects in the MTHFR gene, and deficiencies of vitamin B12 and folate.23
Evidence from clinical trials for the beneficial role of a regimen of supplemental folate, vitamin B6, and vitamin B12 on CVD risk is lacking. Christen and colleagues found that daily supplementation with 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg of vitamin B12 for seven years reduced homocysteine levels by 18% in 300 women with three or more risk factors for coronary heart disease but did not affect other cardiovascular inflammatory biomarkers.24
Another study by Kataria and colleagues demonstrated that daily supplementation of 2 mg of folic acid, 25 mg of vitamin B6, and 0.5 mg of vitamin B12 resulted in an 11% reduction in risk of vascular death among 8,513 stroke patients but had no effect on cardiovascular risk.25
In addition, treatment for four weeks with oral doses of 1.8 g/day of N-acetylcysteine, a precursor to the antioxidant glutathione, has been found to lower blood homocysteine levels by reducing cellular oxidation, but has not consistently reduced the risk of cardiac events in cardiovascular patients.26,27
The reasons for the lack of protective effects of folic acid, vitamin B6, and vitamin B12 on inflammatory biomarkers and risk of CVD are unclear. It is possible that the optimal synergistic dosages of these vitamins have not yet been identified or that compounds produced during the metabolism of homocysteine may increase inflammation and override the beneficial effects of supplementation.26
Diabetes
Elevated plasma homocysteine has been associated with an increased risk of insulin resistance and type 2 diabetes. Homocysteine has been shown to block the activation of insulin receptors, resulting in decreased glucose uptake by cells, hyperglycemia, and compensatory hyperinsulinemia.2
Elevated blood homocysteine levels were significantly related to rates of type 2 diabetes among 5,000 adult patients enrolled in 25 multinational prospective and case-control studies. A review of these studies found that patients with type 2 diabetes had significantly higher blood homocysteine levels and greater rates of retinopathy and neuropathy compared with patients with normal blood homocysteine levels. Damage to pancreatic beta cells by oxidized homocysteine has been suggested as a possible mechanism for the increased risk of diabetes.28
Elevated maternal homocysteine levels during pregnancy have been associated with low birth weight, which is an established risk factor for type 2 diabetes. Pregnant women with elevated homocysteine levels have an increased risk of gestational diabetes, miscarriage, preeclampsia, and premature delivery.29 Additionally, infants born to women with folate and vitamin B12 deficiencies and elevated blood homocysteine levels were found to have an increased risk of developing insulin resistance by age 6, compared with women with values in the normal range.2
Daily supplementation with 5 to 10 mg of folic acid for up to 12 weeks significantly lowered plasma homocysteine levels among 426 patients with type 2 diabetes. A nonsignificant reduction in plasma C-reactive protein levels was observed. However, an effect of supplemental folic acid on two other biomarkers of inflammation—tumor necrosis factor and interleukin-6—was not observed.30
Chronic Kidney Disease
An elevated plasma level of homocysteine is an independent risk factor for chronic kidney disease and is linked with declining kidney function as the disease progresses.31 Over 85% of patients with chronic kidney disease have hyperhomocysteinemia, and plasma homocysteine levels in patients with end-stage renal disease are three to five times higher than those of normal individuals.32 A significant increase in plasma homocysteine levels, from 12.9 mmol/L in stage 1 to 23.68 mmol/L in stages 4 through 5, was observed in 132 patients with chronic kidney disease.31
Within the kidney, homocysteineinduced inflammation and oxidative stress lead to elevated urinary albumin levels (microalbuminuria) and injury to the podocytes, the renal filtration cells. The prolonged exposure of renal cells to oxidative damage impairs the ability of the tubules to transport, absorb, and excrete electrolytes and water, and leads to fibrosis and loss of kidney function. Hyperhomocysteinemia is strongly associated with end-stage renal disease and its progression to renal dialysis.2,32
A study by Chen and colleagues explored the relationship between serum homocysteine levels and the incidence of chronic kidney disease among 79,416 adults. Hyperhomocysteinemia was defined as a homocysteine level greater than 15 umol/L, and chronic kidney disease was defined as a decrease in glomerular filtration rate <60 ml/min/1.73 m2 at baseline or hematuria and proteinuria for more than three months. Individuals with hyperhomocysteinemia experienced twice the incidence of chronic kidney disease than individuals with normal homocysteine levels.33
Supplementation with folic acid, vitamin B6, and vitamin B12 has been shown to lower plasma homocysteine levels in patients with chronic kidney disease. A five-year clinical trial in which 619 hemodialysis patients who received 2.5 mg of folic acid, 1 mg of vitamin B12, and 50 mg of vitamin B6 daily had significantly lower plasma levels of homocysteine. However, there was no reduction in cardiovascular risk within this group. Similarly, another study of folic acid supplementation among predialysis patients found reductions in plasma homocysteine levels but no change in endothelial function or reduced risk of cardiovascular events.34
Neurological Disorders
Elevated plasma homocysteine levels contribute to oxidative stress, inflammation, and atrophy within the brain, characteristic of neurological disorders.35
Stroke is a leading cause of death worldwide. Hyperhomocysteinemia may increase the risk of stroke by causing the development of plaque and thrombi (blood clots) inside cranial and cardiac blood vessels. A large study (N=9,888) found that individuals who experienced ischemic stroke had significantly higher levels of plasma homocysteine compared with healthy controls. A striking finding of this analysis was that even mild hyperhomocysteinemia (<30 umol/L) increased the probability of stroke. The authors noted that the damaging effects of homocysteine begin to appear between 8 and 10 µmol/L.36
Hyperhomocysteinemia may increase the risk of Alzheimer’s disease by compromising the integrity of the bloodbrain barrier, allowing toxic substances to pass through to brain tissue. Hyperhomocysteinemia may also contribute to the formation of tau protein and amyloid plaques within the brain, biomarkers of Alzheimer’s disease.35
Autism spectrum disorder is a bioneurological developmental disability that affects one in 36 children in the United States.37 Infants diagnosed with autism spectrum disorder have decreased plasma levels of methionine and cysteine and increased levels of homocysteine, compared with infants without autism. These findings suggest that defects in the remethylation and transsulfuration pathways may contribute to the elevated homocysteine levels observed in children with autism spectrum disorder.35
Epidemiological studies have found that individuals experiencing major depressive disorder and bipolar disorder have elevated plasma levels of homocysteine and deficient plasma levels of folate and vitamin B12.35 The effect of reducing plasma homocysteine levels on the symptomology of these neurological disorders has not been well-studied, and clinical trials investigating associations between folic acid and vitamin B12 supplementation and mood disorders have provided inconsistent results. A meta-analysis of 16 clinical trials (N=6,276) did not find a significant effect of vitamin supplementation on depressive symptoms.38 However, a review of five clinical trials (N=6,507) by da Silva and colleagues found that supplementation with folic acid, vitamin B6, and vitamin B12 was effective in preventing depression in patients recovering from stroke.39 Supplementation with folic acid, vitamin B6, and vitamin B12 may improve symptoms of depression and bipolar disorder among patients with these disorders by increasing the absorption and utilization of mood-stabilizing medications.35
Hyperhomocysteinemia is also associated with an increased risk of schizophrenia. A meta-analysis of data from eight case-control studies demonstrated that every 5-mm increase in plasma homocysteine was associated with a 70% increase in the risk of schizophrenia.35 Hyperhomocysteinemia may contribute to the development of schizophrenia due to folate and vitamin B12 deficiencies, which impair the body’s production of the antioxidant glutathione. Glutathione depletion has been observed among individuals with schizophrenia and may contribute to the pathogenic changes in the brain that occur in neurodegenerative diseases.40
Huang noted that a rare mutation of the MTRR gene, which controls production of the MTR enzyme, was found in an adult patient with schizophrenia who had an elevated plasma homocysteine level.41 Supplementation with B vitamins improved negative symptoms in patients with schizophrenia who were diagnosed with genetic defects affecting folate metabolism.39
A randomized clinical trial in which 42 patients experiencing schizophrenia were treated daily for three months with 2 mg folate, 25 mg vitamin B6, and 400 ug vitamin B12 reported significant reductions in plasma homocysteine levels and improved clinical symptoms compared with control participants.35
Liu and colleagues looked at the effects of vitamin supplementation in a metaanalysis of 16 studies of healthy adults with normal or mildly elevated plasma homocysteine levels (N=1,369). A daily combination of 1 mg of folate, 7.2 mg of vitamin B6, and 20 ug of vitamin B12 effectively reduced plasma homocysteine levels in these individuals.42
Many patients with inborn errors of metabolism that affect homocysteine metabolism, such as CBS deficiency, require a low-protein diet in which methionine-rich foods such as meat, fish, eggs, and dairy foods are restricted. Specific recommendations for dietary protein restrictions for metabolic disorders like CBS deficiency do not exist. However, guidelines published by the World Health Organization and Food and Agriculture Organization in 2007 can be used to guide the total protein requirements.43 Protein restrictions should be personalized and based on an individual’s plasma homocysteine level. Supplementation with methionine-free formulas of amino acids is often needed to avoid protein deficiencies. In addition, supplementation with 100 to 200 mg/kg of betaine may effectively reduce homocysteine levels in these individuals.21
Putting It Into Practice
Hyperhomocysteinemia is a serious medical condition that contributes to the risk of many chronic diseases. Nutrition professionals can help their clients reduce their elevated blood levels of homocysteine levels. Understanding and interpreting the laboratory tests that screen for hyperhomocysteinemia will facilitate identification of individuals with the disorder. Recommendations for vitamin supplementation should be individualized and based on blood levels of folate, vitamin B6, and vitamin B12. Individuals with metabolic disorders requiring protein restriction should be educated about the types and amounts of protein foods they can eat, and about supplemental nutritional formulas that will boost protein intake. Teaching clients about dietary sources of folate, vitamin B6, B12, and betaine is key, particularly for vegans and individuals adhering to restricted diets. The most important dietary recommendations for patients experiencing hyperhomocysteinemia are the following:
1. Increase intake of folate-rich foods: leafy greens, legumes, fortified cereals, and citrus fruit. Although orange juice is also a good source of folate, consumption should be limited to 4 oz per day to prevent rapid increases in blood sugar levels.
2. Add foods rich in vitamin B12: fish, lean meat and poultry, eggs, dairy foods, and fortified cereals, or nutritional yeast and fortified plant-based milks for vegans and vegetarians.
3. Include foods rich in vitamin B6: starchy vegetables, bananas, poultry, and fortified cereals.
4. Eat more foods rich in betaine: beets, spinach, and whole grains.
5. Avoid excessive intake of alcohol and highly processed foods.
— Mary Franz, MS, RDN, is a freelance health and science writer.
Learning Objectives
After completing this continuing education course, nutrition professionals should be better able to:
1. Explain the synthesis and regulation of homocysteine in the body.
2. Identify the effects of elevated plasma homocysteine on chronic disease risk.
3. Provide clients with hyperhomocysteinemia with individualized dietary guidelines for restoring normal plasma homocysteine levels.
Examination
1. What is the role of methionine synthase in homocysteine metabolism?
a. It converts dietary choline to betaine.
b. It catalyzes the remethylation of homocysteine to methionine.
c. It promotes B12 absorption in the gut by activating intrinsic factor.
d. It converts inactive B6 to pyridoxal 5’-phosphate.
2. Which of the following is the most important regulator of homocysteine metabolism?
a. The availability of methylcobalamin
b. The conversion of pyridoxamine to pyridoxal 5’-phosphate
c. The level of S-adenosyl-L-methionine in cells
d. The amount of protein in the diet
3. Which metabolic condition causes homocystinuria?
a. A mutation of the cystathionine beta-synthase gene
b. A deficiency of the enzyme needed to metabolize cysteine
c. Excessive hydrogen sulfide production by the liver
d. The binding of homocysteine to lysine in cellular proteins
4. Which of the following is true about hyperhomocysteinemia during pregnancy?
a. Hyperhomocysteinemia during pregnancy poses no risk to the fetus.
b. Infants born to pregnant women with elevated homocysteine levels are at risk of low birth weight.
c. An elevated blood homocysteine level in a pregnant woman has no effect on her blood glucose level.
d. A pregnant woman with hyperhomocysteinemia should limit her weight gain to avoid developing insulin resistance.
5. What is the purpose of the transsulfuration pathway?
a. It converts glutathione to cysteine.
b. It supplies methyl groups to proteins.
c. It catalyzes dietary choline to betaine.
d. It converts homocysteine to cysteine.
6. How does hyperhomocysteinemia increase the risk of chronic kidney disease?
a. Homocysteine damages podocytes and causes fibrosis within renal tubules.
b. It decreases the glomerular filtration rate to < 90 ml/min/1.73 m2.
c. It interferes with reabsorption of sodium in the renal tubules.
d. It increases the urinary excretion of pyridoxine.
7. What did a large study that looked at hyperhomocysteinemia and risk of stroke find?
a. Mild hyperhomocysteinemia was not associated with higher rates of stroke.
b. Even mild elevations in plasma homocysteine levels increased the risk of stroke.
c. The association between hyperhomocysteinemia and risk of stroke was seen only in elderly individuals.
d. All of the study participants had folate deficiencies.
8. Research suggests that a 5 umol/L increase in plasma homocysteine increases the risk of coronary heart disease by what percentage?
a. 40%
b. 30%
c. 20%
d. 10%
9. Which statement about dietary protein restriction is true for individuals with homocystinuria?
a. All patients with homocystinuria should receive the same protein restriction.
b. Small amounts of methionine-rich foods are permitted.
c. Supplementation with folate, vitamin B6, and vitamin B12 is unnecessary.
d. There are no specific guidelines for protein-restricted diets for homocystinuria.
10. What combination of vitamin supplements has been found to reduce plasma homocysteine levels in healthy adults?
a. 1 mg of folic acid, 7.2 mg of vitamin B6, and 20 μg of vitamin B12
b. 400 μg of folic acid and 400 μg of vitamin B12
c. 800 μg of folic acid and 6 mg of vitamin B6
d. 400 μg of folic acid, 6 μg of vitamin B12, and 3 g of betaine
References
1. Wu DF, Yin RX, Deng JL. Homocysteine, hypertension, and H-type hypertension. Eur J Prev Cardiol. 2024;31(9):1092-1103.
2. Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab (Lond). 2017;14:78.
3. Son P, Lewis L. Hyperhomocysteinemia. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK554408/.
4. Folate. National Institutes of Health, Office of Dietary Supplements website. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/#h8. Updated November 30, 2022. Accessed March 2, 2025.
5. Vitamin B6. National Institutes of Health, Office of Dietary Supplements website. https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/#h2. Updated June 16, 2023. Accessed March 2, 2025.
6. Vitamin B12. National Institutes of Health, Office of Dietary Supplements website. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/. Updated March 26, 2024. Accessed March 2, 2025.
7. Al Mutairi F. Hyperhomocysteinemia. clinical insights. J Cent Nerv Syst Dis. 2020;12:1179573520962230.
8. Salama AA. Nutritional management of hyperhomocysteinemia. In: Waly MI, ed. Nutritional Management and Metabolic Aspect of Hyperhomocysteinemia. Springer, Cham; 2021:199-213. https://doi.org/10.1007/978-3-030-57839-8_16
9. McCaddon A, Miller JW. Homocysteine – a retrospective and prospective appraisal. Front Nutr. 2023;10:1179807.
10. Dobrijević D, Pastor K, Nastić N, et al. Betaine as a functional ingredient: metabolism, health-promoting attributes, food sources, applications and analysis method. Molecules. 2023;28(12):4824.
11. Sellos-Moura M, Glavin F, Lapidus D, Evans K, Lew CR, Irwin DE. Prevalence, characteristics, and costs of diagnosed homocystinuria, elevated homocysteine, and phenylketonuria in the United States: a retrospective claims-based comparison. BMC Health Serv Res. 2020;20(1):183.
12. CBS (homocysteinemia/cystathionine beta-synthase deficiency). Newborn Screening Info website. https://www.newbornscreening.info/cbs-homocystinemia-cystathionine-beta-synthase-deficiency/. Updated March 1, 2023. Accessed February 25, 2025.
13. Ruiz-Mercado M, Vargas MT, Pérez de Soto I, et al. Methionine synthase reductase deficiency (Cb1E): A report of two patients and a novel mutation. Hematology. 2016;21(3):193-197.
14. Hübner V, Hannibal L, Janzen N, Grünert SC, Freisinger P. Methionine adenosyltransferase I/III deficiency detected by newborn screening. Genes. 2022;13(7):1163.
15. Pfeiffer CM, Sternberg MR, Zhang M, et al. Folate status in the US population 20 y after the introduction of folic acid fortification. Am J Clin Nutr. 2019;110(5):1088-1097.
16. Tang Y, Xu M, Kuo S-M. Factors contributing to the high prevalence of vitamin B6 deficiency in US: a systematic review. J Hum Nutr. 2018;2(1):58-64.
17. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040.
18. Cario H, Smith DEC, Blom H, et al. Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease. Am J Hum Genet. 2011;88(2):226-231.
19. Plecko B, Mills P. PNPO deficiency. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2025. https://www.ncbi.nlm.nih.gov/books/NBK581452/
20. Koklesova L, Mazurakova A, Samec M, et al. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021;12(4):477-505.
21. González-Lamuño D, Arrieta-Blanco FJ, Fuentes ED, et al. Hyperhomocysteinemia in adult patients: a treatable metabolic condition. Nutrients. 2024;16(1):135.
22. Wang B, Mo X, Wu Z, Guan X. Systematic review and meta-analysis of the correlation between plasma homocysteine levels and coronary heart disease. J Thorac Dis. 2022;14(3):646-653.
23. Habib SS, Al-Khlaiwi T, Almushawah A, Alsomali A, Habib SA. Homocysteine as a predictor and prognostic marker of atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Eur Rev Med Pharmaco Sci. 2023;27(18):8598-8608.
24. Christen WG, Cook NR, Van Denburgh M, Zaharris E, Albert CM, Manson JE. Effect of combined treatment with folic acid, vitamin B6, and vitamin B12 on plasma biomarkers of inflammation and endothelial dysfunction in women. J Am Heart Assoc. 2018;7(11):e008517.
25. Kataria N, Yadav P, Kumar R, et al. Effect of vitamin B6, B9, and B12 supplementation on homocysteine level and cardiovascular outcomes in stroke patients: a meta-analysis of randomized controlled trials. Cureus. 2021;13(5):e14958.
26. Paganelli F, Mottola G, Fromonot J, et al. Hyperhomocysteinemia and cardiovascular disease: is the adenosinergic system the missing link? Int J Mol Sci. 2021;22(4):1690.
27. Hildebrandt W, Sauer R, Bonaterra G, Dugi KA, Edler L, Kinscherf R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am J Clin Nutr. 2015;102(5):1014-1024.
28. Wang JX, You DY, Wang HP, et al. Association between homocysteine and type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Diabetes Dev Ctries. 2021;41:553-562.
29. Thakur P, Bhalerao A. High homocysteine levels during pregnancy and its association with placenta-mediated complications: a scoping review. Cureus. 2023;15(2):e35244.
30. Mokgalaboni K, Mashaba GR, Phoswa WN, Lebelo SL. Folic acid supplementation on inflammation and homocysteine in type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Nutr Diabetes. 2024;14(1):22.
31. Chen CH, Huang SC, Yeh EL, Lin PC, Tsai SF, Huang YC. Indoxyl sulfate, homocysteine, and antioxidant capacities in patients at different stages of chronic kidney disease. Nutr Res Pract. 2022;16(4):464-475.
32. Long Y, Nie J. Homocysteine in renal injury. Kidney Dis (Basel). 2016;2(2):80-87.
33. Chen W, Feng J, Ji P, Liu Y, Wan H, Zhang J. Association of hyperhomocysteinemia and chronic kidney disease in the general population: a systematic review and meta-analysis. BMC Nephrol. 2023;24(1):247.
34. Badri S, Vahdat S, Seirafian S, Pourfarzam M, Gholipur-Shahraki T, Ataei S. Homocysteine-lowering interventions in chronic kidney disease. J Res Pharm Pract. 2021;10(3):114-124.
35. Cordaro M, Siracusa R, Fusco R, Cuzzocrea S, Di Paola R, Impellizzeri D. Involvements of hyperhomocysteinemia in neurological disorders. Metabolites. 2021;11(1):37.
36. Pinzon RT, Wijaya VO, Veronica V. The role of homocysteine as a risk factor of ischemic stroke: a systematic review and meta-analysis. Front Neurol. 2023;14:1144584.
37. Autism fact sheet. National Autism Association website. https://nationalautismassociation.org/resources/autism-fact-sheet/. Accessed March 11, 2025.
38. Markun S, Gravestock I, Jäger L, Roseman T, Pichierri G, Burgstaller JM. Effects of vitamin B12 supplementation on cognitive function, depressive symptoms, and fatigue: a systematic review, meta-analysis, and meta-regression. Nutrients. 2021;13(3):923.
39. da Silva TR. Can supplementing vitamin B12 improve mental health outcomes?: a literature review. Br J Community Nurs. 2024;29(3):137-146.
40. Zhilyaeva TV, Piatoikina AS, Bavrina AP, et al. Homocysteine in schizophrenia: independent pathogenic factor with prooxidant activity or integral marker of other biochemical disturbances? Schizophr Res Treatment. 2021;2021:7721760.
41. Huang CC. Rare variants in the MTRR gene, 66CG and 524TT cause hyperhomocysteinemia and folic acid deficiency linked to schizophrenia. Front Psychiatry. 2024;15:1353308.
42. Liu C , Yao H, Wang F. Effect of nutritional supplements for reducing homocysteine levels in healthy adults: a systematic review and network meta-analysis of randomized trials [published online February 17, 2025]. Nutr Rev. doi: 10.1093/nutrit/nuae191.
43. Morris AMM, Kožich V, Santra S, et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2017;40(1):49-74.
