Today’s Dietitian
Vol. 18 No. 5 P. 46
Learn About the Diet, the Medical Conditions It’s Used to Treat, and Its Mechanism of Action.
Suggested CDR Learning Codes: 5000, 5070, 5080, 5300
Suggested CDR Performance Indicators: 8.1.5, 8.3.6
CPE Level: 2
Take this course and earn 2 CEUs on our Continuing Education Learning Library
The ketogenic diet (KD) is a high-fat, low-carbohydrate diet with adequate protein that’s a nonpharmacologic treatment for refractory epilepsy.1,2 Although the use of the KD was first reported in 1921 by Russell M. Wilder, MD, at Mayo Clinic,1,3 Hippocrates established in the 5th century BC that fasting reduced seizure activity.1,3 Then, in the early 20th century, two French neurologists reported a decrease in seizures in patients who completed a four-day fast.1 Wilder used this knowledge to develop a diet that mimicked the metabolic effects of fasting by inducing ketosis (a state in the body where ketone bodies replace glucose as the major source of energy), demonstrating that the effects of fasting could be maintained through a diet.
A small number of neurologists used Wilder’s original KD over the course of the next half-century with few to no changes to the protocol and no clinical studies of the patient population.1 During this time there were great advances in antiepileptic drugs, which contributed to the stagnation of studies of the diet.4 However, in the early 1990s there was a resurgence of the use and study of the KD.3,4 For the past 20 years, the KD has become increasingly popular as families seek alternative therapies with fewer side effects and neurologists offer the diet to a broader range of epilepsy patients. This growing popularity has led to randomized controlled trials that have demonstrated the diet’s efficacy in several different pediatric populations.3 In general, the majority of those patients who initiate the KD experience a significant (>50%) reduction in seizures.1
There are now several different types of the KD that allow for more dietary treatment choices for patients. There also have been changes to the way the diet is initiated, which has helped to ease the diet’s burden.3
This continuing education course reviews the KD, the medical conditions it’s used to treat, and its mechanisms of action. The various types of the KD, the composition of the diet, common side effects of the diet, and the outcomes and effectiveness of the diet also are discussed.
Use of the KD
The KD is primarily used to treat medication-resistant/nonresponsive pediatric epilepsy.5 Epilepsy is defined as “a tendency to have recurring, unprovoked seizures.” Recurring generally is defined as at least two seizures over an individual’s lifetime.6,7 Seizures can be defined as “sudden, brief attacks of altered consciousness; motor, sensory, cognitive, psychic, or autonomic disturbances; or inappropriate behavior caused by abnormal, excessive, or synchronous neuronal activity in the brain.”7
Seizures are categorized into two groups: partial/focal seizures and generalized seizures.8 Partial or focal seizures occur in only one part of the brain and can be simple (no loss of consciousness) or complex (loss of consciousness). Generalized seizures occur in both sides of the brain, often causing loss of consciousness.8
Epilepsy is the most common neurological disorder in the world, affecting 3% of the general global population.7 In Western populations, 1 in 2,000 people has epilepsy.7 The incidence of epilepsy is the highest in the first 12 months of life, and then again after 60 years of age.7 There are multiple epilepsy syndromes, each with different causes, presentations, treatments, and outcomes.5,7,9
There’s no definitive research regarding which epileptic syndromes the KD most effectively treats. It has been theorized that the KD doesn’t differ in efficacy based on type of seizure or syndrome.10 During the past decade, more studies have provided additional information on the KD and the populations who benefit from it.
The 2008 recommendations of the International Ketogenic Diet Study Group indicate that the KD may be efficacious for the following types of epilepsy: infantile spasms, tuberous sclerosis complex, myoclonic-astatic epilepsy (Doose syndrome), severe myoclonic epilepsy of infancy (Dravet syndrome), and Rett syndrome.11,12 Other studies have reported better response to the KD in infantile spasms than in complex partial seizures; two studies in particular have shown that the KD is effective in severe myoclonic epilepsy in infancy.1,4
The 2012 National Institute for Clinical Excellence guidelines recommend that all children with epilepsy who haven’t responded to antiepileptic drugs should be considered for a trial of the KD.5 This recommendation has been reiterated by several other reports, stating that due to increased access to the KD and its new, less restrictive forms, the diet should be considered as alternative therapy for children with refractory epilepsy as well as individuals in older populations.7,9
The KD also has been used to treat two rare metabolic genetic disorders: pyruvate dehydrogenase deficiency (PDH) and glucose transporter-1 deficiency syndrome (GLUT1). The use of the diet in these populations is a first-line therapy option and can be life saving.11-14 Patients with PDH and GLUT1 likely will have to follow the KD for life. This course focuses only on the use of the KD in the intractable epilepsy population.
Mechanisms of Action
The specific mechanisms of the KD and how fasting results in a decrease or cessation of seizures isn’t known. However, major advancements have been made in understanding the diet’s many complex effects on the central nervous system. More research on the mechanisms of action is needed to further validate the KD’s effectiveness.12 With more information about its mechanisms, the KD might be deployed in a more focused way and could be further refined.
What’s known is that when there’s a large decrease in carbohydrate consumption, glucose utilization is reduced. When this happens, the liver uses fatty acids to produce ketone bodies, such as beta-hydroxybutyrate (BHB) and acetoacetate. These are then used in place of glucose to provide energy for cellular metabolism.15 The KD replaces glucose as the major source of energy with ketone bodies, thus inducing a state of ketosis.16 In the body, a large amount of energy is used to fuel neurons. In individuals on the KD diet, the elevated ketone bodies inhibit neuronal excitability, slowing the firing rates of neurons, which could decrease seizure activity.15,16
An important question regarding the mechanism of action of the KD, one that currently remains unanswered, is whether it’s the production of ketone bodies or the decrease in glucose that drives the diet’s efficacy.15,16 If ketone bodies are the main contributors to decreased seizures, one would expect to see a correlation with serum ketone concentration and effectiveness.15 However, only one study to date has shown improved outcomes with higher serum BHB levels (an indicator of ketone bodies), while several other studies have shown no correlation.15
In addition, newer forms of the KD, which don’t always lead to ketosis, have been shown to be effective in decreasing seizures.16 Decreased glucose and insulin as a driving factor also is supported by the fact that the diet’s benefits are reversed when glucose is intravenously infused.16
There are several theories on the specific mechanisms of action of the KD that won’t be discussed in this article but demonstrate the substantial progress that has been made in the last decade regarding the multifaceted mechanism of action of the KD.
Types of KD
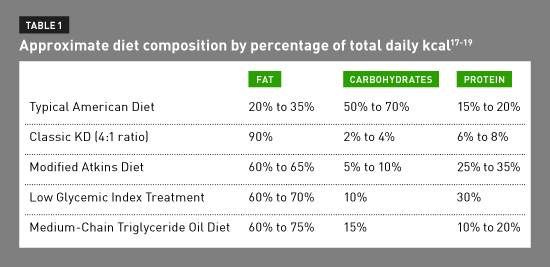
There are a variety of types of the KD. These are summarized in Table 1.
Classic KD
The classic KD is the original diet developed more than a century ago and is still the most prevalent type used today.4 Johns Hopkins Hospital published the original protocol on initiation and maintenance of the diet and advocated for its use only in patients aged 1 to 15.4 The KD isn’t used as often in infants, because it requires more careful monitoring and observation, but it can be highly effective if needed. Its use in infants is becoming more widespread now that a liquid formulation is available.4 The use of the diet in adults is still rare, but it’s being piloted in several clinics; however, due to poorer adherence, preliminary data suggest outcomes aren’t as good as those in children.20,21
Initiation of the classic KD is an evolving process that has been altered in many clinics over the last two decades. The original protocol was to begin the diet after a period of fasting and fluid restrictions lasting approximately two days to induce a rapid ketotic state.4,10 However, this can be physically and emotionally difficult for patients. After a randomized clinical trial showed that gradual initiation of the diet was as effective as a fasting initiation and had fewer side effects, many clinics moved to the better tolerated gradual initiation.4,22,23
The classic KD most often is initiated during a three- to seven-day hospital stay.2,4,23 During this hospitalization, the patients are observed for hypoglycemia, dehydration, and acidosis. This is also a time to provide extensive education to parents and patients on calculating the diet, preparing foods, and identifying carbohydrate-containing foods. This guidance is critical to the success of the KD.2,4,23 While most clinics prefer hospitalization during initiation, there are some clinics that support initiation of the KD in an outpatient setting.4
The most accepted distribution of nutrients in the classic KD is the 4:1 ratio, or four parts fat (90%) and one part carbohydrates and protein (10%) (see Table 1).1,22,24,25 It also has been proposed that the classic KD is efficacious at a lower ratio of 3:1, or closer to 80% fat, 15% protein, and 5% carbohydrates by weight.4 This lower ratio is a good choice to ensure patients get adequate protein for growth.4 Recently, clinics have begun starting patients at the lower 3:1 ratio. If there aren’t satisfactory results, the higher ratios of 3.5:1 or 4:1 are used.4
All ratios of the classic KD require the families and/or patients to weigh, measure, and calculate all food and the majority of beverages consumed, to the gram. Even very small errors can cause a return of seizures.23 With the original protocol, patients are expected to follow the diet for approximately three years, which includes a period of about 12 months when patients are slowly weaned off the diet.4 Some patients choose to stay on the diet longer if their seizures worsen during the weaning process. Some have been on the diet for a decade or longer.
To date, there have been several large studies showing the efficacy of the classic KD. The results of these studies have varied, but most show that after six to 12 months, between 50% to 70% of patients remain on the diet, 45% to 70% show a 50% reduction in seizures, and 10% to 30% have a more than 90% reduction in seizures, with some becoming seizure free.4,12,26,27 These results also have been shown to be maintained after three to six years on the diet.4 This efficacy was improved in patients given only the ketogenic liquid formula; 100% of these patients showed improvement in seizure frequency and severity.28
A recent study showed that while 24% of patients put on the KD experienced freedom from seizures for at least 28 days, there’s only a 3% chance that those same patients will never again have seizures.29 Overall, however, patients didn’t return to the same frequency of seizures and remained much improved from their baseline.29 While the diet’s effects aren’t always maintained when a patient is weaned from the KD, benefits do persist for the majority of patients when compared with their pre-KD period.4
The classic KD is appropriate for those patients who receive formula feedings and those who can tolerate strictly regimented diets. Small children often are tolerant of this type of diet. The caregiver also must be willing to prepare foods that follow very precise diet orders and weigh all foods to one-tenth of a gram. This diet also works well for caregivers that prefer detailed instructions and ample guidance.30
Modified Atkins KD
The Modified Atkins Diet (MAD) was first introduced in 2003 but received its official name in a 2006 publication from Johns Hopkins University School of Medicine to distinguish it from the popular Atkins diet, on which it was based in part.12,27 The main differences between the MAD and the Atkins diet is that the strict restriction of carbohydrates, referred to as the induction phase in the Atkins diet, is maintained throughout the duration of the MAD; high-fat foods are encouraged; and the primary goal is seizure reduction, not weight loss.27 The MAD consists of approximately 60% fat, 30% protein, and 10% carbohydrates (see Table 1).
On this diet, net carbohydrates, which are calculated by subtracting fiber from total carbohydrates, are counted.23,27,28 The net carbohydrates are restricted to 10 to 20 g per day but can be increased slowly after the initiation period based on results and patients’ tolerance.27,28 Calories, fluids, and protein are unrestricted on this diet and food doesn’t need to be measured.23,27,28 Due to these lighter restrictions, the diet can be initiated on an outpatient basis.
These modifications have been helpful for patients who found the classic KD unpalatable or too strict.4,23,25,27 The MAD has been reported to be well tolerated in children, adolescents, and adults and to have fewer side effects than the classic KD.4,23,25,31 Even though this diet is seemingly easier to administer and follow than the classic KD and patients can eat more typical foods, an extensive support system of nurses, physicians, and dietitians is important to maintain the diet because it’s still restricted and therefore requires clinical monitoring.23
In recent literature, the MAD’s efficacy has been shown to be similar to that of the classic KD.23,25,27 Several studies have shown a greater than 50% reduction in seizures in 40% to 50% of patients on the MAD after three to six months.2,21,23,31 Also, approximately 35% to 40% of patients on the MAD showed a greater than 90% reduction in seizures, with similar numbers becoming seizure-free as compared with those on the classic KD.2,27,31
In one study that compared the MAD to the classic KD, there was no statistically significant difference between the two results; however, there was an overall trend of a higher response rate in the classic KD.31 Kossoff and colleagues showed that there’s better efficacy with 10 g compared with 20 g of carbohydrates per day, and that there’s a subset of patients who will find improved outcomes when they transition to the classic KD from the MAD.23,27
Caregivers and patients are given less direction (no prescribed meal plans) with the MAD. As a result, this may work best for those who are more independent and confident. This diet also may be appropriate for patients unable to tolerate strictly regimented diet plans, such as some teenagers and adults. The lack of meal plans on the MAD may be a disadvantage for patients who benefit from more structure. In their own center, the authors have noticed that many parents under- or overfeed their children on the MAD. For that reason, portion size instructions and close weight monitoring are often necessary.30
Low Glycemic Index Treatment KD
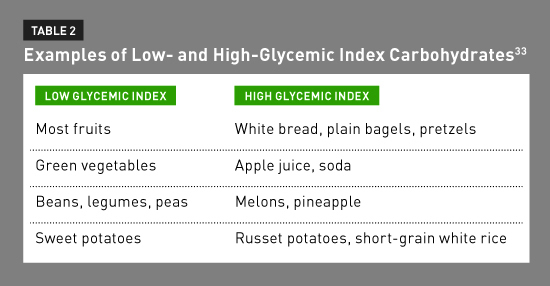
The low glycemic index treatment (LGIT) KD was introduced in 2005 at Massachusetts General Hospital for Children and is similar to the MAD in that only net carbohydrates are counted.32 This diet was developed based on the hypothesis that maintaining low, stable insulin levels contributes to the effectiveness of the KD.32 The glycemic index ranks foods based on how quickly blood glucose rises after the ingestion of a particular food item.33
In the LGIT, only low glycemic index (LGI) carbohydrates (glycemic index <50) are permitted and a greater amount of total net carbohydrates (40 to 60 g/day) are allowed (see Table 1). LGI carbohydrates cause blood sugar and insulin levels to rise slowly.33 Table 2 displays examples of low and high glycemic index foods. There are few studies on the effectiveness of the LGIT. The largest study of 76 epileptic children on the LGIT showed a greater than 50% reduction in seizures in approximately one-half of the children.34
The MAD and LGIT diets are simpler to administer than the classic KD because patients aren’t required to weigh and calculate all foods and beverages. The MAD and LGIT also have the advantages of an outpatient initiation, the ability to consume larger portions of protein and carbohydrates, and more flexibility in meal planning.30
Because of its relaxed restrictions, the LGIT (as well as the MAD) can be much easier for patients to implement and integrate into their daily lives. For example, on the LGIT, patients can go to a restaurant and simply eat a hot dog without the roll. Whereas on the classic KD, patients need to bring a scale to the restaurant, calculate the grams of hot dog they’re allowed for the meal, and then weigh it before they can eat it. Due to the flexibility of both the MAD and LGIT, these diets may be the better choice for teenagers and adults.35
Medium-Chain Triglyceride Oil Diet
This diet was introduced in the 1970s at the University of Chicago and uses medium-chain triglyceride (MCT) oil to induce ketosis. MCTs go directly to the liver and are absorbed more effectively and produce more ketones per gram of fat compared with long-chain triglycerides (LCTs).17 Since ketone production is greater with MCTs, this diet has the advantage of reducing the amount of fat needed to 60% of total calories (See Table 1).
In the traditional version of this diet, all of the fat comes from MCT oil. However, this diet is limited by the gastrointestinal (GI) side effects that large doses of MCT oil can cause, such as loose stools, vomiting, and abdominal pain.17 It’s difficult for patients to tolerate large amounts of MCTs. Due to these side effects, a modified version of this diet has been tried with one-half of the fat (30% of total calories) coming from MCTs and the other one-half from LCTs. To boost ketones, MCT oil also can be substituted for regular oil in the classic KD. Studies have shown that the MCT oil diet can be as effective as the classic KD.17,36
While the original version of the MCT oil diet is difficult to tolerate, the modified version of the diet with one-half the fat is a good option for patients who can’t tolerate larger amounts of fat in their diets.
Ketogenic Meals
The KD can be administered in many forms: as oral meals, formula for infants, or tube feedings.
Oral Diets
The classic KD requires that all meals adhere to strict guidelines the RD provides for the total amount of calories as well as the grams of carbohydrates, protein, and fat in each meal and snack. Before starting the diet, families are asked to complete a three-day dietary record for the patient.
The diet record is analyzed for average calorie intake and used along with the recommended daily intake (RDI) of calories for age and weight to determine daily caloric requirements. Growth, blood glucose readings, and hunger levels are monitored over time and considered when determining calorie increases and decreases. Children are expected to have no change in percent of ideal body weight.37 Age-appropriate weight gain and linear growth is the goal, and caloric intake is adjusted when possible to attain this goal.
On the classic KD, every meal must be calculated to fit the nutrient guidelines the RD provides and food ingested must first be weighed to the tenth of a gram. This is a large commitment as meal preparation can be quite time-consuming. Families bring their scales on vacation, to restaurants, and picnics. Each meal usually contains heavy whipping cream (as a source of fat), fat (butter, margarine, mayonnaise, or oil), a small amount of protein (meats, eggs, cheese, nuts), and an even smaller amount of carbohydrates (fruits, vegetables).
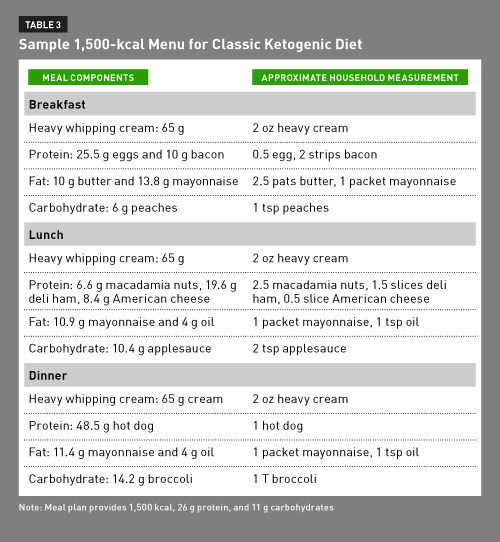
Special ketogenic versions of typically high-carbohydrate foods, such as pancakes, muffins, and cupcakes, can be prepared using ingredients such as almond flour, soy flour, ground nuts, and eggs. Patients are encouraged to use oil as the fat source as it’s more heart-healthy than butter or mayonnaise.38 Many children drink the oil along with their meals. Each meal must be finished in its entirety to maintain the ketogenic ratio. Table 3 provides a sample daily 1,500-kcal menu.
Previously, fluid intake was restricted on the KD. However, in recent years these restrictions have been liberalized.35 Today, increased fluid intake often is encouraged to help prevent constipation and kidney stones.22
The KD is very low in carbohydrates. Patients vary in their sensitivity to additional carbohydrates; in some, minimal amounts of carbohydrates beyond the prescribed diet can cause seizures. As such, even the carbohydrate content of medications should be taken into consideration; pediatric medications that are taken orally usually are sweetened, which adds a noticeable amount of carbohydrates to the total daily intake. Occasionally, children will ingest foods that aren’t part of their planned meals. In those cases, extra fat can be given to cover the extra carbohydrates the child received. It’s also important that children on the diet use only low-carbohydrate toothpaste.
For patients on the MAD, LGIT, or MCT oil diets, meals aren’t as restrictive. They’re still high in fat and low in carbohydrates, but the limitations aren’t as extreme. Since fiber isn’t counted toward the restriction in carbohydrates, the best carbohydrate choices are high in fiber. On the MAD and LGIT diet, patients or caregivers need to count the net carbohydrates (total carbohydrates minus fiber) ingested and aim to reach a prescribed goal of net carbohydrates by the end of the day. Patients also are encouraged to increase daily fat intake in an amount recommended by an RD. Protein isn’t measured, but it does need to be limited to prevent excess weight gain or failure to achieve good ketosis.
Enteral Diets
In the United States, there are two commercially available enteral formulas for KD patients. KetoCal is a cow’s milk protein-based formula manufactured by Nutricia North America. The formula comes in a powdered, mixable form and a ready-made liquid version with a ketogenic ratio of 4:1. The powdered version of KetoCal is available in 3:1 and 4:1 ketogenic ratios.
If a different ratio is needed, a source of carbohydrates (such as another formula, whole milk, or apple juice) or a source of fat (such as Microlipid by Nestlé Nutrition or MCT oil) can be added to the formula for individualized plans. For example, to reduce the KetoCal 4:1 liquid to a lower ratio such as 3.5:1, 22 g of apple juice can be added to 500 g of KetoCal 4:1 liquid. The powdered form also can be used in cooking to make carbohydrate replacements such as pancakes, muffins, and pizza.
Abbott Nutrition produces Ross Carbohydrate Free (RCF), a soy protein-based formula option. By itself, RCF is a 1.8:1 ratio. Ketogenic formula regimens often are written as recipes that include multiple ingredients. To transform the RCF into a higher ratio, such as 3:1 or 4:1, a source of fat, such as Microlipid, can be added. Ingredients such as apple juice, Pedialyte (Abbott Nutrition), or Duocal (Nutricia North America) may be added to the formula recipe to add carbohydrates. See Figure 1 for a sample KD formula recipe.

Supplemental protein powders also can be used to increase the protein content of a formula regimen. Water can be added to the formula recipe to meet fluid requirements and achieve the desired caloric density. When preparing formula, each ingredient should be weighed accurately on a gram scale. Prepared formula should be refrigerated and used within 24 hours.
Nutrient Content of the KD
There’s a significant difference in the nutrient composition of the KD compared with the recommended amounts of nutrients for the healthy individual. The recommended nutrient distribution for healthy individuals by total calories is a moderate carbohydrate (45% to 65%); adequate protein (10% to 35%); and lower fat (20% to 35%) diet.39 In comparison, the KD is a very high-fat (60% to 90%), adequate protein (5% to 10%), and very low-carbohydrate (3% to 10%) diet.1,22,24,25
The classic KD is based on a 4:1 ratio, with four parts fat to every one part protein and carbohydrates combined, or about 90% of the total caloric intake as fat.24 Recently, with the development of different types of ketogenic diets, the ratio can range a bit, some 3:1 or even 2:1, with the fat being as low as 60% to 65% of total calories.24
In all types of the KD, however, the carbohydrate content is kept significantly lower than that in an average diet. The actual amounts of carbohydrates recommended vary based on the type of KD. Traditionally, those on the 4:1 ratio call for less than 10 g/day of carbohydrates, or about 3% of calories.22,25 Some types allow carbohydrates up to about 10% of total calories per day.25
With the KD, protein intake is kept as low as possible while meeting the World Health Organization’s minimum requirements for age, or about 1 g/kg of body weight, or equal to around 7% of total calories.1,22,26 In the original KD protocol, calories were restricted to 85% to 90% of estimated daily requirements.3 Some patients are still on restricted-calorie diets; however, more often patients are started on a slight calorie restriction, but by the end of the trial are at 100% of estimated caloric requirements, with some patients taking full calories from the beginning.1,24 Lastly, the original KD protocol restricted fluids to 1,200 to 1,500 mL per day. This guideline also has been modified and fluids are now typically unrestricted to prevent constipation and dehydration.1
Electrolytes and Micronutrients
Due to the restricted carbohydrates and protein on the KD, vitamins, minerals, and electrolytes from nutrient-rich food sources are very limited.40 Micronutrient supplementation is required for all patients while they are on the KD. Sodium, potassium, and chloride are frequently supplemented with regular table salt or reduced-sodium salts such as Morton Lite Salt.
On the classic KD, supplement regimens are prescribed to meet at least 100% of the RDI for all vitamins and minerals. Vitamins and minerals dietitians should focus on when planning supplement regimens include vitamin D, calcium, magnesium, selenium, and zinc.22 There are supplements such as FruitiVits by Vitaflo USA and Phlexy-Vits by Nutricia North America geared specifically to patients on restrictive therapeutic diets. If a patient prefers not to take these supplements, an alternate regimen can be prescribed that involves multiple supplements (see Figure 2).
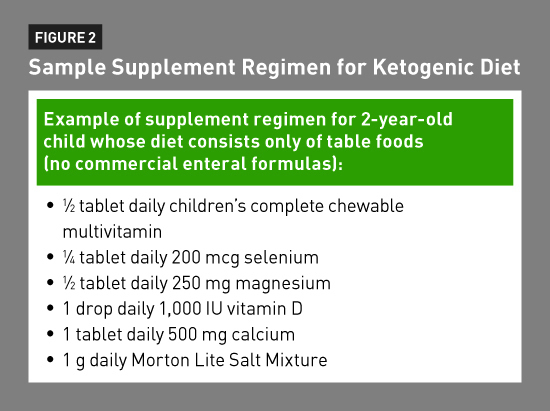
For patients on the MAD, LGIT, or MCT diets, the supplement regimens usually are simpler and include only an over-the-counter complete multivitamin with minerals and possibly a calcium supplement. Serum levels of electrolytes, magnesium, phosphorus, zinc, selenium, and vitamin D should be reviewed for all KD patients every three to six months.22
Nutritional Consequences/Side Effects
The KD is a medically prescribed diet with consequences and side effects. RDs need to be aware of these issues and provide education to patients about potential challenges.
Hyperlipidemia
One side effect of the KD patients often experience is elevated lipid levels. Significantly increased total and LDL cholesterol and triglycerides combined with significantly decreased HDL cholesterol have been reported in up to 50% of patients.41,42 In some cases, hyperlipidemia has been shown to occur within days of KD initiation.43 Kang and colleagues reported elevated cholesterol in 14.7% of patients and elevated triglycerides in 27.1% of patients.44 Hyperlipidemia isn’t a reason to discontinue the KD; however, lipids should be monitored every three months.22
Nutrition counseling can be beneficial for patients on oral diets. It has been shown that modifying the type of dietary fat by reducing saturated fat can improve the lipid profile.38 It also has been demonstrated that improving the types of fat in commercially available ketogenic formulas can reduce hyperlipidemia.45
Linear Growth
There have been several studies indicating that the KD can negatively affect linear growth.22,38,46 This may be due to the restriction of calories and protein. Neal and colleagues analyzed the growth of children on the MCT oil diet and the classic KD.47 The authors found that both groups had decreased height and weight gain velocity. This may be related to a reduction in insulinlike growth factor-1 (IGF-1) and growth hormone.
In another study, IGF-1 was measured twice—once before the patients started the KD and then a second time one year later—and was found to have greatly decreased.48 Catch-up growth has been documented after patients discontinue the KD.49 When growth is slowed, increased protein and/or increased calories as well as ratio reduction can be considered.22
Bone Health
Bone health also can be a concern for KD patients. Before starting the KD, epilepsy patients are at higher risk of fractures due to increased falls caused by seizures and because antiepileptic drugs interfere with calcium and vitamin D absorption.22,50 Although the KD reduces drug usage, it also can exacerbate bone health issues. Loss of bone mineral content after patients have started the KD has been documented.51
It’s theorized that the acidic environment caused by ketones may be involved in reduced bone mineral accretion.51 Increased fractures also have been reported with long-term use of the KD.46 To reduce fracture risk, some KD centers require dual-energy X-ray absorptiometry scans every two years for KD patients and provide calcium and vitamin D supplements.22,35
GI Issues
As many as 75% of KD patients experience adverse GI symptoms.22 Most patients with severe neurological impairment have delayed intestinal transit time and gastroesophageal reflux disorder (GERD) before starting the KD.52 The KD usually aggravates these issues. With little fiber in the diet, constipation is common.
Children are encouraged to increase fluid intake to alleviate some of these symptoms.22,35 Many KD patients take laxatives regularly to help control constipation,22,35 which can cause vomiting and loss of appetite. GERD is also a common side effect of the KD. Not surprisingly, in a diet that’s up to 90% fat the food is slow to leave the stomach and can cause GERD, retching, and vomiting. Many children are prescribed H2-blockers or proton pump inhibitors to alleviate this problem.35
Acidosis
During initiation of the KD, many children experience metabolic acidosis.18,35 The ketone bodies created during ketosis are acids and increase the level of acid load in the body. Some antiepileptic drugs also contribute to acidosis.35
These children are given bicarbonate supplements to normalize their acidosis and usually continue taking them for their entire time on the KD.35 The serum carbon dioxide level, which indicates acidosis, is monitored every three months for at least the patient’s first year on the diet.35
Kidney Stones
Kidney stones, or nephrolithiasis, is a side effect found in 5% to 25% of KD patients.44,46,53 Formation of stones may be a result of the vast metabolic changes caused by the KD.22 The stones have been found to be correlated with elevated levels of calcium in the urine.53
Increased fluid intake helps to reduce the risk.22 In addition, oral citrate supplementation can be recommended to neutralize the pH of the urine, making it less acidic.53 Some clinicians recommend preventive use of citrates.35 Citrates also bind to calcium in urine, preventing buildup of calcium crystals.
Final Thoughts
The KD is a very high-fat, low-protein, and minimal-carbohydrate diet that’s an exceptionally effective method for treating medication-resistant seizures. When medications have failed, the KD is likely to be more effective than introducing another medication.35 Approximately two-thirds of patients who begin the KD experience a significant reduction in seizures.54,55
Why and how the diet works is still something of a medical mystery, in part because of its complexity in altering many different metabolic pathways. The KD switches the body’s primary fuel source from glucose to ketones and maintains low, stable glucose and insulin levels. All three of these factors likely contribute to how the KD reduces seizures.
The KD can be difficult to adhere to since it isn’t easy to create palatable oral meals in which 90% of the calories come from fat. Cream, mayonnaise, butter, or oil must be a part of every meal. To make the diet more tolerable for patients, less restrictive versions have been developed: the LGIT diet, MAD, and MCT oil diet. There are good options for administering the diet via gastrostomy tube. The classic KD is still the most commonly used form, but all of the diets have their advantages and disadvantages. Each may work better with a different population. The diet does have significant side effects, and it’s important that a medical team follow patients on the KD regularly.
RDs play an important role in the initiation and monitoring of the KD as well as providing ongoing patient support. RDs are crucial in determining calorie needs, macronutrient guidelines, and supplementation recommendations. They provide ongoing support in implementing a challenging diet by assisting with meal planning, feeding issues, recipes, and product recommendations. RDs also are essential in monitoring the patient’s growth and lab work to minimize side effects. The KD presents a unique opportunity for RDs to be involved in implementing an effective treatment for a serious illness.
— Rebecca Randall, MS, RD, is a clinical coordinator for a pharmaceutical company who previously worked with patients on the ketogenic diet.
— Sue Groveman, MS, RD, LDN, is a clinical pediatric dietitian at the Children’s Hospital of Philadelphia who currently works with patients on the ketogenic diet.
Learning Objectives
After completing this continuing education course, nutrition professionals will be better able to:
1. Assess the potential mechanisms of action of the ketogenic diet (KD).
2. Discuss the medical conditions the KD is used to treat.
3. Differentiate and evaluate the four types of KDs.
4. Explain at least three side effects of the KD.
CPE Monthly Examination
1. Which serum level test is used as an indicator of ketone bodies?
a. Serum beta-hydroxybutyrate levels
b. Serum acetoacetate levels
c. Serum glutamate levels
d. Blood glucose levels
2. The medical conditions that the ketogenic diet (KD) is known to effectively treat are:
a. Doose Syndrome and Lennox-Gastaut syndrome
b. Pyruvate dehydrogenase deficiency, glucose transporter-1 deficiency syndrome, and epilepsy
c. Early infantile epileptic encephalopathy or early myoclonic encephalopathy
d. All forms of childhood epilepsy
3. Which of the following represents the macronutrient distribution of the KD?
a. 45% to 65% carbohydrate, 10% to 35% protein, and 20% to 35% fat
b. 70% to 85% carbohydrate, 10% to 20% protein, and 5% to 20% fat
c. 3% to 10% carbohydrate, 5% to 10% protein, and 60% to 90% fat
d. 20% to 30% carbohydrate, 30% to 40% protein, and 30% to 50% fat
4. Which of the following is the type of KD that requires all food to be weighed and measured, requires an inpatient hospital stay when patients first start the diet, previously required fasting with fluid restrictions, and is the most prevalent type of the diet used today?
a. Modified Atkins KD (MAD)
b. Low glycemic index treatment (LGIT) KD
c. Medium-chain triglyceride (MCT) KD
d. Classic KD
5. Which type of KD keeps patients on a low net carbohydrate intake for the duration of the diet, makes high-fat foods mandatory, and can be started on an outpatient basis with fewer documented side effects than those caused by previous forms of the diet?
a. Classic KD
b. MAD
c. LGIT
d. MCT KD
6. Which of the following are side effects of the KD?
a. Kidney stones, hyperlipidemia, gastroesophageal reflux disorder (GERD)
b. Hyperlipidemia, fatigue, blurry vision
c. Hyperthyroidism, high cholesterol, hyperlipidemia
d. GERD, stomach upset, skin reactions
7. Why is MCT oil used in the KD?
a. It’s absorbed more quickly.
b. It’s easy to drink.
c. It doesn’t cause hyperlipidemia.
d. It increases ketones.
8. Electrolytes are supplemented using which of the following?
a. Table salt or lower-sodium Morton Lite Salt
b. Gatorade
c. Pedialyte
d. Cured meats
9. All foods eaten in the classic KD should be measured in what way?
a. Using household measuring spoons and cups
b. By eye
c. By serving size
d. Using a gram scale
10. What is given to normalize acidosis in patients on the KD?
a. Histamine-2 blockers
b. Bicarbonate supplements
c. Proton pump inhibitors
d. Fluid
References
1. Caraballo RH, Vining E. Ketogenic diet. In: Stefan H, Theodore WH, eds. Handbook of Clinical Neurology: Epilepsy Part II: Treatment. 1st ed. Philadelphia, PA: Elsevier; 2012:783-793.
2. Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet in infantile spasms refractory to first-line treatment. Seizure. 2012;21(1):45-48.
3. Kossoff EH. Nonpharmacological approaches: diet and neurostimulation. In: Dulac O, Lassonde M, Sarnat HB, eds. Handbook of Clinical Neurology: Pediatric Neurology Part I. 1st ed. Philadelphia, PA: Elsevier; 2013:803-808.
4. Kang HC, Kim HD. Diet therapy in refractory pediatric epilepsy: increased efficacy and tolerability. Epileptic Disord. 2006;8(4):309-316.
5. Appleton RE, Freeman A, Cross JH. Diagnosis and management of the epilepsies in children: a summary of the partial update of the 2012 NICE epilepsy guideline. Arch Dis Child. 2012;97(12):1073-1076.
6. Arts WF, Geerts AT. When to start drug treatment for childhood epilepsy: The clinical-epidemiological evidence. Eur J Paediatr Neurol. 2009;13(2):93-101.
7. Elger CE, Schmidt D. Modern management of epilepsy: a practical approach. Epilepsy Behav. 2008;12(4):501-539.
8. Seizure and epilepsy: hope through research. National Institutes of Health National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/epilepsy/detail_epilepsy.htm#252623109. Updated November 20, 2015.
9. Cross JH. New research with diets and epilepsy. J Child Neurol. 2013;28(8):970-974.
10. Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv Pediatr. 2010;57(1):315-329.
11. Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6(2):406-414.
12. Rho JM, Stafstrom CE. The ketogenic diet: what has science taught us? Epilepsy Res. 2012;100(3):210-217.
13. Graham JM. GLUT1 deficiency syndrome as a cause of encephalopathy that includes cognitive disability, treatment-resistant infantile epilepsy and a complex movement disorder. Eur J Med Genet. 2012;55(5):332-334.
14. Klepper J, Diefenbach S, Kohlschütter A, Voit T. Effects of the ketogenic diet in the glucose transporter 1 deficiency syndrome. Prostaglandins Leukot Essent Fatty Acids. 2004;70(3):321-327.
15. Danial NN, Hartman AL, Stafstrom CE, Thio LL. How does the ketogenic diet work? Four potential mechanisms. J Child Neurol. 2013;28(8):1027-1033.
16. Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36(1):32-40.
17. Neal EG, Chaffe H, Schwartz RH. A randomized trial of classic and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50(5):1109-1117.
18. Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn). 2013;19(3 Epilepsy):756-766.
19. Pfeifer HH, Lyczkowski DA, Thiele EA. Low glycemic index treatment: implementation and new insights into efficacy. Epilepsia. 2008;49(8):42-45.
20. Mosek A, Natour H, Neufeld MY, Shiff Y, Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure. 2009;18(1):30-33.
21. Weber S, Mølgaard C, Taudorf K, Uldall P. Modified Atkins diet to children and adolescents with medical intractable epilepsy. Seizure. 2009;18(4):237-240.
22. Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100(3):261-266.
23. Joshi SM, Singh RK, Shellhaas RA. Advanced treatments for childhood epilepsy beyond antiseizure medications. JAMA Pediatr. 2013;167(1):76-83.
24. Bansal S, Cramp L, Blalock D, Zelleke T, Carpenter J, Kao A. The ketogenic diet: initiation at goal calories versus gradual caloric advancement. Pediatr Neurol. 2014;50(1):26-30.
25. Porta N, Vallée L, Boutry E, et al. Comparison of seizure reduction and serum fatty acid levels after receiving the ketogenic and modified Atkins diet. Seizure. 2009;18(5):359-364.
26. Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500-506.
27. Kossoff EH, Cervenka MC, Henry BJ, Haney CA, Turner Z. A decade of the modified Atkins diet (2003-2013): results, insights, and future directions. Epilepsy Behav. 2013;29(3):437-442.
28. El-Rashidy OF, Nassar MF, Abdel-Hamid IA, et al. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand. 2013;128(6):402-408.
29. Taub KS, Kessler SK, Bergqvist CG. Risk of seizure recurrence after achieving initial seizure freedom on the ketogenic diet. Epilepsia. 2014;55(4):579-583.
30. Whitmer E, Reither JL, Kossof EH. Fighting Back With Fat. New York, NY: Demos Health; 2013.
31. Miranda MJ, Mortensen M, Povlsen JH, Nielsen H, Beniczky S. Danish study of a modified Atkins diet for medically intractable epilepsy in children: can we achieve the same results as with the classic ketogenic diet? Seizure. 2011;20(2):151-155.
32. Pfeifer HH, Thiele EA. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurol. 2005;65(11):1810-1812.
33. Foster-Powell K, Miller JB. International tables of glycemic index. Am J Clin Nutr. 1995;62(4):871S-890S.
34. Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50(5):1118-1126.
35. Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50(2):304-317.
36. Schwartz RH, Eaton J, Bower BD, Aynsley-Green A. Ketogenic diets in the treatment of epilepsy: short-term clinical effects. Dev Med Child Neurol. 1989;31(2):145-151.
37. Liu YM, Williams S, Basualdo-Hammond C, Stephens D, Curtis R. A prospective study: growth and nutritional status of children treated with the ketogenic diet. J Am Diet Assoc. 2003;103(6):707-712.
38. Fenton C, Chee CM, Bergqvist AGC. Manipulation of types of fats and cholesterol intake can successfully improve the lipid profile while maintaining the efficacy of the ketogenic diet. Infant Child Adolesc Nutr. 2009;1(6):338-341.
39. Dietary reference intakes (DRIs): recommended dietary allowances and adequate intakes, total water and macronutrients. Institute of Medicine website. http://www.iom.edu/Activities/
Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/New%20Material/
3_RDA%20AI%20AMDR%20Values_Total%20Water%20and%20Macronutr.pdf. Updated July 24, 2013. Accessed October 9, 2014.
40. Zupec-Kania B, Zupanc ML. Long-term management of the ketogenic diet: seizure monitoring, nutrition, and supplementation. Epilepsia. 2008;49(Suppl 8):23-26.
41. Chesney D, Brouhard BH, Wyllie E, Powaski K. Biochemical abnormalities of the ketogenic diet in children. Clin Pediatr. 1999;38(2):107-109.
42. Kwiterovich PO Jr, Vining EP, Pyzik P, Skolasky R Jr, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA. 2003;290(7):912-920.
43. Dekaban AS. Plasma lipids in epileptic children treated with the high fat diet. Arch Neurol. 1966;15(2):177-184.
44. Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45(9):1116-1123.
45. Groveman SA, Fenton C, Randall R, Chee CM, Bergqvist AG. Ketogenic diet patients’ lipid profiles improved with KetoCal 4:1 liquid. Infant Child Adolesc Nutr. 2015;7(3):157-161.
46. Groesbeck DK, Bluml RM, Kossoff E. H. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48(12):978-981.
47. Neal EG, Chaffe H, Edwards N, Lawson MS, Schwartz RH, Cross JH. Growth of children on classic and medium-chain triglyceride ketogenic diets. Pediatrics. 2008;122(2):e334-e340.
48. Spulber G, Spulber S, Hagenäs L, Amark P, Dahlin, M. Growth dependence on insulin-like growth factor-1 during the ketogenic diet. Epilepsia. 2009;50(2):297-303.
49. Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia. 2010;51(7):1277-1282.
50. Shellhaas RA, Joshi SM. Vitamin D and bone health among children with epilepsy. Pediatr Neurol. 2010;42(6):385-393.
51. Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88(6):1678-1684.
52. Del Giudice E, Staiano A, Capano G, et al. Gastrointestinal manifestations in children with cerebral palsy. Brain Dev. 1999;21(5):307-311.
53. Sampath AE, Kossoff H, Furth SL, Pyzik PL, Vining EP. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol. 2007;22(4):375-378.
54. Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol. 2006;21(3):193-198.
55. Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics. 2000;105(4):E46.


