 CPE Monthly: Eye Health and Nutrition
CPE Monthly: Eye Health and Nutrition
By Kathleen Searles, MS, RDN, LD
Today’s Dietitian
Vol. 25 No. 1 P. 40
CPE Level 2
Take this course and earn 2 CEUs on our Continuing Education Learning Library
Impaired vision is a major public health problem, with more than 250 million persons experiencing vision loss worldwide and more than 3.4 million Americans aged 40 and older experiencing visual impairment.1,2 Vision loss is one of the 10 most common causes of disability in the United States, potentially leaving individuals unable to drive, read, or travel independently. Moreover, it may require specialized equipment as vision deteriorates. People with visual impairment are more likely to suffer from depression, diabetes, hearing impairment, stroke, falls, and cognitive decline.2
This continuing education course explores the role of nutrition in visual development and healthy vision. Nutritional factors in prevention and treatment of age-related macular degeneration (AMD), glaucoma, cataract, dry eye disease (DED), and visual complications of diabetes will be reviewed.
Overview of the Eye Structure
The human eye is part of a visual system that includes the eye, neural pathways to the brain, and areas of the brain for interpreting visual signals. The pupil regulates light entering through the cornea. The lens focuses light on the retina, which consists of blood vessels, rods, and cones. These photoreceptors translate light into neural impulses. The millions of cones are concentrated in the macula, allowing for sharp, detailed central vision and color vision. The surrounding rods are responsible for night vision, peripheral vision, and motion detection3 (See Figure).
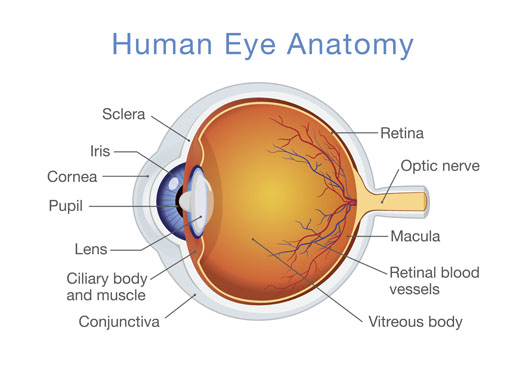
Key Nutrients for Maintaining Eye Health
Vitamin A, which plays an essential role in vision, is involved in cell differentiation in the cornea and conjunctival membrane, functions as an antioxidant, and helps convert light into neural signals. Vitamin A is necessary for the formation of rhodopsin, the photoreceptive pigment responsible for low-light vision.4,5 Vitamin A deficiency, which is the leading cause of preventable blindness worldwide,4,6 presents as xerophthalmia, drying of the conjunctiva and cornea and night blindness due to inadequate rhodopsin, the earliest symptom.5,6
There’s a low risk of vitamin A deficiency in developed countries, except in postbariatric surgery patients.7 Vitamin A is obtained from the diet as preformed vitamin A (retinol or retinyl esters from animal sources such as fish oils, eggs, dairy products, and liver) or carotenoids, primarily β-carotene, from plant sources (dark green or yellow/orange vegetables, fruits, and oils).4,6 As a fat-soluble vitamin, it’s better absorbed with dietary fat.
Vitamin A circulates in the body in a one-to-one complex with retinol-binding protein.6 Conditions that affect formation of retinol-binding protein, such as proteinuria, protein malnutrition, or zinc deficiency, can contribute to vitamin A deficiency.4
The human eye is susceptible to damage from oxidative stress. Eye tissue has a high oxygen consumption and a high concentration of polyunsaturated fatty acids (PUFAs), and it’s exposed to high-energy visible light, which contributes to the formation of reactive oxygen species that can damage cells.1 Nutrients that function as antioxidants can help mitigate this damage, but excessive intake isn’t helpful.8
Dietary lutein (Lu) and zeaxanthin (Zx) function in the macular pigment of the retina to shield the eye from light damage.8,9 Macular pigment filters blue light and acts in an antioxidant, anti-inflammatory role.9 Lu and Zx are obtained from dietary sources such as green leafy vegetables (kale, spinach, broccoli, and lettuce), egg yolks, wheat, yellow peppers, and yellow corn.8,10 Dietary fat enhances absorption of Lu and Zx. These carotenoids are transported in the body by lipoproteins, with theoretical implications that manipulating lipoprotein levels may affect Lu and Zx levels in the retina.10
Long chain PUFA intake also may impact eye health. DHA, an omega-3 fatty acid found in the photoreceptor cells, provides light protection, helps regenerate corneal nerve cells, and maintains cell membranes.8,11 In addition, omega-3 fatty acids provide anti-inflammatory effects.12 A balanced intake of omega-6 and omega-3 fatty acids seems to be most beneficial, with an ideal ratio of 4:1 and an acceptable ratio of <10:1.8,12 Dietary sources of omega-3 fatty acids include flaxseeds, flaxseed oil, fish oil, walnuts, chia seeds, hemp seeds, and fatty fish.13,14
Nutrients with antioxidant properties or roles in antioxidant enzyme systems also are being investigated. These include vitamins C, D, and E; zinc; copper; selenium; glutathione; and dietary flavonoids.8,13,15,16 There’s also evidence showing associations between deficiencies in the various B vitamins and eye disorders.17-19
Maternal and Infant Nutrition and Eye Development
Because of its role in cell differentiation, vitamin A is an essential nutrient for proper eye development.5 Maternal nutrition plays a key role in the development of vision; ingested maternal vitamin A reaches the infant via the placenta.4,20
The earliest manifestation of maternal vitamin A deficiency is night blindness. Pregnant women with poor diets, infections, diabetes, or gestational diabetes are most at risk.4,6 Those living in less-developed countries also face higher risk.
Excessive vitamin A can be teratogenic, so it’s important to ensure adequate but not excessive intake. In 2013, the World Health Organization recommended against routine supplementation, except in places where vitamin A deficiency is a known public health issue.4
Carotenoids are deposited in eye tissue beginning at about 20 weeks gestation.20 During the third trimester of pregnancy, uptake of carotenoids from the placenta accelerates, and fetal rhodopsin levels increase dramatically.9,21 Lu and Zx also play a role in the developing retina. Macular pigment begins accumulating prenatally, with levels detectible at birth and continuing to accumulate until age 7.9
Research is ongoing about the roles of choline, DHA, and arachidonic acid in fetal eye development.22,23 Fortification of infant formulas with DHA has been associated with improvements in visual acuity in full-term infants.24 The third trimester is important for retinal growth when large amounts of DHA are transferred to the fetus via the placenta.9
Retinopathy of prematurity (ROP), the second leading cause of childhood blindness in the United States, is seen in low-birthweight premature infants. Important risk factors for ROP are extremely low birthweight and gestational age ≤ 30 weeks.25 Vitamin A deficiency can increase the risk of ROP.23 Vitamin E supplementation can significantly reduce the risk of severe ROP in very low-birthweight infants but is recommended primarily for infants who are >34 weeks because of the possibility of increased risk of necrotizing enterocolitis.24
Nutritional Factors in Eye Disorders
Age-Related Macular Degeneration
AMD is the most common cause of blindness in industrialized countries. One in three individuals over the age of 80 show signs of AMD.26
There are two types of AMD: dry and wet. In dry AMD, photosensitive cells break down. In wet AMD, abnormal blood vessels form under the retina (angiogenesis) causing blurred vision and impaired central vision.3 Angiogenesis is treated with injections that inhibit vascular endothelial growth factor (VEGF) and control edema.3,20 As AMD advances, lipid deposits (drusen) accumulate in the retina. When widespread, the condition is known as geographic atrophy.26
AMD is associated with multiple lifestyle and genetic risk factors, which vary among ethnicities.20,27 Interpretation of AMD studies may be affected by whether genetic risk has been identified. It has been suggested that individuals with two risk alleles may have limited results from both nutrient supplementation and diet manipulation.28
Age is the most significant risk factor for AMD. Other risk factors include smoking, obesity, exposure to ultraviolet and blue light, poverty, light skin color, light iris color, and possibly female sex.3,10,20 Poor nutrition, including from restrictive diets, has been implicated.10 In particular, a low intake of green leafy vegetables and fruit may increase risk.29
The primary studies on nutrition and AMD are the Age-Related Eye Disease Studies, AREDS and AREDS2. These large (>4,000 participants) studies looked at the roles of antioxidant vitamins, minerals, and carotenoids in reducing AMD incidence and progression.30 Participants were individuals aged 55 to 80 from 11 centers nationwide who were at risk of developing AMD. In the three arms of AREDS, researchers compared the effects of supplemental zinc (80 mg zinc oxide with supplemental copper as 2 mg cupric oxide), supplemental antioxidants (500 mg vitamin C, 400 IU vitamin E, and 15 mg β-carotene), or both on AMD development.1,20,30 These nutrients were chosen for their antioxidant and immune-supporting functions.11 Participants were followed for five years, with follow-up after an additional five years.30 For the antioxidants + zinc and copper treatment arm, there was no preventive effect, but there was a 25% reduction in risk of progression to late stages of AMD compared with placebo.1,20,30 (See Table below.)
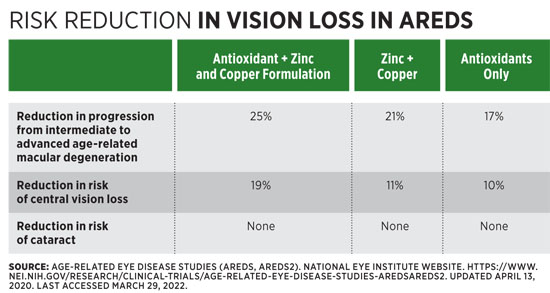
AREDS2 was designed to assess the effect of Lu, Zx, and/or omega-3 PUFA on AMD.20 Some arms of the study used the original AREDS supplement, some used a lower zinc level (25 mg), and some used the supplement without β-carotene.30 An increased risk of lung cancer was found in participants who were smokers or had a history of asbestos exposure who received β-carotene.11 Overall, the findings showed a modest to no-risk reduction for the use of Lu and Zx compared with placebo.26
The Vitamin E, Cataract and Age-Related Maculopathy Study (n=1,204) looked at the preventive effects of supplemental vitamin E. There was no evidence for the general population to use supplemental vitamin E for prevention or to slow progression of AMD.26,31
Merle and colleagues studied intake of B vitamins and progression to geographic atrophy in AMD. After controlling for multiple variables, they found that high dietary folate intake correlated with a reduced risk of progression to geographic atrophy.18 Gopinath and colleagues looked at 1,760 participants aged 55 and older and found that an elevated total homocysteine level was related to increased risk of AMD as were vitamin B12 and folate deficiencies.17
In the Carotenoids Age-Related Eye Disease Study (CAREDS), Moeller and colleagues evaluated 1,787 women aged 50 to 79 who were in the highest and lowest ranges for Lu and Zx intake. They found a strong inverse relationship between intermediate AMD and vegetable intake, especially green vegetables. In healthy women, they found a statistically significant protective effect of higher Lu and Zx intakes.32 CAREDS also found decreased prevalence of early AMD with increased adherence to a Mediterranean diet.28 In a CAREDS study of vitamin D, Millen and colleagues found the highest risk of AMD in those with deficient vitamin D status and two risk alleles for AMD.16
When Gopinath and colleagues examined intake of flavonoids, specifically those found in apples, oranges and orange juice, and tea, they found a protective effect on AMD prevalence. Study participants who consumed at least one serving of oranges (rich sources of the flavonoid hesperidin) per week had 92% reduced odds of late AMD.15
Summarizing results of various studies, Demmig-Adams and colleagues suggest that individuals can lower the risk of AMD by leading a healthy lifestyle and consuming a diet with ample antioxidants, especially Lu, Zx, and omega-3 fatty acids. The ideal diet is calorically appropriate, low glycemic, and limited in trans and saturated fats, with a desirable omega-6:omega-3 fatty acid ratio. In addition, they recommend a multivitamin/mineral supplement containing vitamins C, E, β-carotene, zinc, and copper at RDA levels.8 Gopinath and colleagues also recommend high consumption of dark green leafy vegetables, consuming fish at least twice weekly, and including ample fruits, especially oranges.15
Glaucoma
Glaucoma is a degenerative retinal neuropathy related to oxidative stress, typically with elevated intraocular pressure. 19,33 Risk factors include age over 60, genetics, family history, diabetes, systemic hypotension or hypertension, vasospasm, use of corticosteroids, migraine, obstructive sleep apnea, myopia, and history of eye injury.33 African Caribbean, African American, and Hispanic or Latino individuals are at the greatest risk.33,34 Elevated BMI has been associated with increased intraocular pressure.35
Despite the role of oxidative stress in glaucoma, no clear beneficial role for antioxidant nutrients has emerged.19 In the prospective, population-based Rotterdam Study, those with lower intake of retinol equivalents were at increased risk of primary open-angle glaucoma.36 Ramdas completed a comprehensive systematic review of 46 studies and found no consistent results associating carotenoid intake with glaucoma.13 In a meta-analysis of five studies on vitamin A, two large studies showed a protective effect of dietary intake of retinol equivalents and three showed no significant difference.19
Studies on vitamins and glaucoma have shown varying results for vitamins C and D and thiamin.19 Higher intakes of riboflavin and niacin have been associated with decreased glaucoma risk.36 A review of six studies found no effect of dietary vitamin E intake on glaucoma.19 In a prospective study, Kang and colleagues found no significant relationship between intake of vitamins C and E or carotenoids on the risk of developing primary open-angle glaucoma.35
Elevated intakes or blood levels of minerals including calcium, magnesium, manganese, mercury, and molybdenum have been associated with increased glaucoma risk. For calcium, high supplement intake (800 mg/day or more) is implicated but not dietary calcium. Supplemental selenium used in a randomized controlled trial of cancer patients was associated with increased risk of developing glaucoma. Elevated serum ferritin levels and supplement intake of 18 mg/day or more also have been associated with greater risk.13,37
Data on omega-3 and omega-6 PUFAs show that the ratio of omega-3: omega-6 is more important than intake of either, with higher ratios increasing the risk of developing glaucoma.13,35 Perez de Arcelus and colleagues studied more than 17,000 participants without glaucoma for a median of 8.2 years. They found no significant effect of omega-3 or omega-6 fatty acids, but those in the highest quintile of omega-3:omega-6 had a significantly higher risk of developing glaucoma than those in the lowest quintile.38
Low levels of the antioxidants glutathione and nitric oxide have been noted in some individuals with glaucoma. Dark green leafy vegetables, sources of glutathione and nitric oxide (along with vitamins A, C, and K), have shown a significant protective effect on open-angle glaucoma.13
The Korean National Health and Nutrition Examination Survey found no differences in mean intraocular pressure for any nutrient quartile in participants aged 40 or older.36 The systematic review and meta-analysis by Ramdas and colleagues, found no consistent correlations with glaucoma for plasma or serum levels of any vitamins.19 With few clear trends linking nutrient intake and glaucoma risk, possible guidelines would be to include ample dark green leafy vegetables and sources of vitamins A and C in diets and to avoid excessive supplementation with selenium, calcium, and iron.13,19,37
Cataract
Cataract is a condition of clouding or discoloration of the lens of the eye due to damage by light and oxidation, impairing collection and focusing of light on the retina.3,39 Cataract is the leading cause of visual impairment and blindness worldwide.40
There are four main types of cataract: subcapsular, cortical, nuclear, and mixed (nuclear and cortical).40 The primary treatment for cataract is surgical extraction and lens replacement.3
Risk factors for cataract include age, sex, diabetes, educational status, smoking or tobacco chewing, exposure to sunlight, geographic location in lower latitudes, exposure to ultraviolet light, and moderate to heavy alcohol use. Obesity and associated glucose intolerance, insulin resistance, hyperlipidemia, and hypertension also are risk factors.3,39
Adequate intake of vitamin C, which absorbs ultraviolet light, protects eye tissues from oxidative damage.41 Several large studies have examined vitamin C intake on blood levels. The India Study of Age-Related Eye Disease assessed 1,443 rural Indians over age 50. Those with higher plasma vitamin C had decreased risk of cataract.39,41
The European Eye Study found a significantly reduced prevalence of cataract or cataract surgery in those with high daily intakes of fruits, vegetables, and vitamin C (intakes >107 mg).41 The Nutrition Vision Project (NVP), a subgroup of the Nurses’ Health Study, found a 57% reduction in cataract risk for women aged 60 or younger who consumed at least 363 mg vitamin C daily vs those consuming less than 140 mg per day.39
Braakhuis and colleagues analyzed 14 review articles on nutrients and cataract risk and found that dietary vitamin C or low dose supplements reduced the incidence and progression of cataract, but high doses (≥1,000 mg /day) of supplemental vitamin C increased cataract risk by 21%.40 The Swedish Mammography Cohort Study found a 25% increased risk of cataract extraction in those taking vitamin C supplements for more than 10 years, with the greatest effect among those aged 60 or younger.41
Neither the Women’s Health Study, a randomized double-blinded placebo-controlled study, nor the prospective Physicians’ Health Study (11,545 male participants) found a significant relationship between vitamin C intake and cataract.39 The AREDS study didn’t find any effect of supplementation on development or progression of cataract in well-nourished adults.41
Studies of vitamin E have had variable findings.39 For example, the European Eye Study found high daily intakes to be associated with a significantly decreased prevalence of cataract or cataract surgery.41 The Physicians’ Health Study (400 IU vitamin E every other day) and the Vitamin E, Cataract and Age-Related Maculopathy Study (500 IU daily) found no effect on cataract incidence, progression, or extraction.39,42
In the Beaver Dam Eye Study, participants with the highest quintile of Lu intake had a 50% reduction in cataract compared with the lowest quintile.43 In the Braakhuis and colleagues review, higher intakes of Lu and Zx were associated with a 23% lower incidence of nuclear cataracts and carotenoid intakes of 4 to 6 mg per day with decreased rates of cataract surgery.40 The AREDS2 study and a follow-up by Glaser and colleagues found no significant protective effect of β-carotene, Lu, or Zx for any type of cataract.20,39,44 There may be some benefit to increasing carotenoid intake for those with the lowest baseline intake. However, a review of data from thousands of participants didn’t find a relationship between cataract development and blood levels of β- or α-carotene, lycopene, cryptoxanthin, or total carotenoids.39
As cofactors in the enzymatic activation of antioxidants, B vitamins may mitigate cataract risk.44 A review by Weikel and colleagues found that consuming 2 mcg or greater of riboflavin daily may contribute to decreased risk of cortical and nuclear cataract, especially in individuals with malnutrition. Higher levels of thiamin and niacin generally were associated with lower risk.39 In AREDS, dietary intakes of riboflavin and vitamin B12 were inversely associated with nuclear and cortical cataract. The highest quintiles of vitamin B6, niacin, and vitamin B12 were associated with decreased risk of nuclear cataract. Among those taking Centrum vitamins, the highest folate intakes were associated with increased risk of subscapular cataract.44 However, the European Prospective Study Investigation in Cancer and Nutrition (EPIC) Study found no association between cataract risk and B vitamins other than increased risk with high vitamin B12 intake.39
Lower fasting glucose levels are associated with reduced incidence and progression of cataract. In the NVP study, women who consumed greater than 200 g of carbohydrates were more at risk than those consuming less than 185 g. In the Melbourne Visual Impairment Project (n=3,217), participants without diabetes who consumed more than 181 g of carbohydrates daily had a three-fold greater risk of cortical cataract. In the Blue Mountain Eye Study (BMES) cohort of 933 participants, those consuming diets with the highest glycemic index were more likely to develop cortical cataracts over 10 years. AREDS found a similar trend for nuclear cataract.39
Evidence linking fat intake and cataract is conflicting. EPIC found an increased risk of any cataract with elevated blood levels of saturated fat and cholesterol. The BMES found a 30% decreased risk of cortical cataract with >6.8 g per day of PUFAs. In contrast, the NVP study found a 2.3-fold increased risk of nuclear cataract with higher PUFA intake. In the total Nurses’ Health Study, there was a decreased risk of cataract in those with higher intakes of EPA and DHA, but in the NVP subset there was a greater risk with increased omega-3 PUFA intake.39
Dietary patterns such as vegetarian diets and the Mediterranean diet may reduce cataract risk.13,39 Current evidence suggests risk reduction can be achieved with a healthy lifestyle including physical activity, no smoking, limited ultraviolet light exposure, and a healthful, low-glycemic index diet rich in fruits, vegetables, and fish.3,39,40
The BMES and EPIC studies associated both low protein intakes and high protein intakes with increased risk of cataract, which suggests a role for decreased meat intake. Recommended daily nutrient targets are 100 g to 150 g of protein and 135 mg of vitamin C. The Clinical Trial of Nutritional Supplements and Age-Related Cataract suggests taking a multivitamin to reduce the risk of nuclear cataracts.39
Dry Eye Disease
DED is a syndrome of inadequate tears affecting the ocular surface, cornea, conjunctiva, and lacrimal ducts.12,45 Inflammation is caused by inadequate tear production and/or increased evaporation of tears. DED affects 5% to 30% of individuals over age 50, with higher rates in postmenopausal women, contact lens wearers, and those with autoimmune conditions.12 It also can result from significant time spent on computers, tablets, or smartphones.45 Symptoms include blurry vision, light sensitivity, irritation, and burning or itching eyes. It’s treated with artificial tears and topical corticosteroids.12
The Women’s Health Study found that women with higher dietary intakes of omega-3 PUFAs had lower risk of DED. An elevated ratio of omega-6:omega-3 was associated with elevated risk.46 In the double-blinded clinical trial Dry Eye Assessment and Management Study, the group supplemented with EPA and DHA had less risk than the group supplemented with olive oil. Pellegrini and colleagues reported on two recent meta-analyses of randomized controlled trials, concluding that omega-3 fatty acids are effective in improving DED.12
As mentioned, vitamin A adequacy is important to prevent xerophthalmia. In Western countries, inadequate vitamin A intake is most likely associated with alcohol use disorders, cystic fibrosis, or postbariatric surgery. Other nutrients being studied include vitamin C, vitamin D, selenium, lactoferrin, curcumin, anthocyanins, and flavonoids.12
Evidence-based nutritional treatment for DED consists of adequate omega-3 PUFAs and correcting any existing vitamin deficiencies.12
Diabetes and Eye Health
With regard to diabetes, diabetic retinopathy (DR) often is a concern. DR is associated with microvascular damage due to elevated blood glucose. Newer evidence points to the role of inflammation in damaging retinal neural cells early in the course of DR.43,47
The leading cause of preventable blindness among working adults, DR affects about one-third of those with diabetes.43 The prevalence and severity of DR is greater among Hispanics, African-Caribbeans, Native Americans, and Indo-Asians.48,49 Some evidence shows a risk of worsening DR postbariatric surgery.50
DR progresses through two stages: nonproliferative DR and proliferative DR. In nonproliferative DR, inflammation leads to vascular and neuronal damage in the retina.43,47 Proliferative DR involves angiogenesis similar to wet AMD.51 In both forms, impaired vascularization can cause diabetic macular edema.43
Treatment for DR includes laser therapy, glucocorticoids, and anti-VEGF drugs. Other helpful medications include angiotensin II receptor-blocking drugs and statins. Fenofibrate with statins have slowed progression of DR, with further research ongoing.43,48,52,53
Most analyses have found no relationship between antioxidant intake and DR.52 In one small study (n=67), a supplement (vitamins C, D3, and E; zinc; EPA; DHA; Zx; and Lu, along with other antioxidants) led to significantly improved visual function and macular pigment optical density after six months.49 Higher-plasma Lu levels and high macular pigment optical density are associated with a lower risk of DR development or progression.43 Elevated blood cholesterol, LDL cholesterol, and triglycerides have been associated with the progression of DR, proliferative DR, and diabetic macular edema.48
The cornerstone for DR management is controlling glucose, blood pressure, and plasma lipids. Achieving good glycemic control early in the course of diabetes leads to the best outcomes.48 The PREDIMED study found a decreased incidence of DR with a Mediterranean diet.51 Future directions may include roles for antioxidant nutrients, new pharmaceutical applications, and gene therapy.49,52
Cortical cataracts occur two to five times more frequently in persons with diabetes. Diabetic cortical cataracts appear at an early age and progress faster than those in people without diabetes. The byproducts of high blood glucose levels create oxidative stress and damage cells in the lens.40,41 Those with diabetes also are nearly twice as likely to develop open-angle glaucoma.53
Putting It Into Practice
Good eye health depends on good nutrition. RDs can assist clients to move toward higher Healthy Eating Index scores and diet patterns, such as the Mediterranean diet, which have been associated with protection against AMD, DR, cataract, and glaucoma.28,51,54 Clients can be assisted to decrease red meats and increase intakes of vegetables (especially leafy greens), fruits, whole grains, fish, nuts, and legumes.11,17,51,55,56 A variety of nutrients consumed as food is more effective than supplementing with isolated nutrients.40,56
To address the important role of nutrition in prenatal eye development, RDs should assist pregnant women with appropriate weight gain and nutrition to support full-term delivery and adequate birth weight. Pregnant women should be screened for adequate intakes of vitamin A, carotenoids, choline, and DHA, and assisted to meet target intake with dietary changes or supplementation.4,9,20,22
RDs also have a role in DR prevention by supporting clients with diabetes in maintaining good glycemic control, managing hypertension, and controlling lipid levels.48 Appropriate dietary goals would be a Mediterranean-style eating pattern and including dietary carotenoids and omega-3 PUFAs.44,51
Working with optometrists and ophthalmologists to support eye health with good nutrition presents an intriguing opportunity for interprofessional practice, as physicians may feel unprepared to offer detailed nutrition advice. As part of an interdisciplinary team, RDs can help create a more complete health care experience for clients.57
— Kathleen Searles, MS, RDN, LD, is a nutrition consultant in private practice.
Learning Objectives
After completing this continuing education course, nutrition professionals should be better able to:
1. Evaluate the role of vitamin A in normal eye development and vision.
2. Discuss four eye disorders and the dietary or nutrient factors involved in their prevention or treatment.
3. Describe the mechanism of diabetic retinopathy and counsel clients on the best dietary interventions for prevention.
Exam
1. What is xerophthalmia?
a. Dry eye disease
b. Vitamin A deficiency disease
c. A precursor to cataract
d. A complication of diabetic retinopathy
2. If you’re counseling a pregnant woman who complains of poor night vision, what dietary counseling approach is most appropriate?
a. Evaluate vitamin E status and recommend adding more vitamin E-rich foods to the diet
b. Recommend the Mediterranean diet
c. Assess vitamin A status and consider supplementation
d. Ensure adequate intake of DHA
3. Which nutrition pattern is most likely to be associated with decreased risk of cataract?
a. High intake of antioxidants, especially Lu, Zx, and omega-3 fatty acids
b. Adequate omega-3 consumption and correction of any vitamin deficiencies
c. A low-glycemic diet rich in fruits and vegetables with adequate protein and vitamin C
d. A diet featuring ample dark green leafy vegetables, good sources of vitamins A and C, and the absence of supplemental selenium and iron
4. Which nutrition pattern is most likely to be associated with decreased risk of glaucoma?
a. High intake of antioxidants, especially Lu, Zx, and omega-3 fatty acids
b. Adequate omega-3 consumption and correction of any vitamin deficiencies
c. A low-glycemic diet rich in fruits and vegetables with adequate protein and vitamin C
d. A diet featuring ample dark green leafy vegetables, good sources of vitamins A and C, and the absence of supplemental selenium and iron
5. Which nutrition pattern is most likely to be associated with decreased risk of age-related macular degeneration (AMD)?
a. High intake of antioxidants, especially Lu, Zx, and omega-3 fatty acids
b. Adequate omega-3 consumption and correction of any vitamin deficiencies
c. A low-glycemic diet rich in fruits and vegetables with adequate protein and vitamin C
d. A diet featuring ample dark green leafy vegetables, good sources of vitamins A and C, and the absence of supplemental selenium and iron
6. Which nutrition pattern is most likely to be associated with decreased risk of dry eye disease?
a. High intake of antioxidants, especially Lu, Zx, and omega-3 fatty acids
b. Adequate omega-3 consumption and correction of any vitamin deficiencies
c. A low-glycemic diet rich in fruits and vegetables with adequate protein and vitamin C
d. A diet featuring ample dark green leafy vegetables, good sources of vitamins A and C, and the absence of supplemental selenium and iron
7. What is the specific cause of diabetic retinopathy?
a. Frequent episodes of hypoglycemia in people with diabetes
b. A combination of microvascular and neural cell damage in the retina associated with high blood glucose levels
c. History of low dietary intake of antioxidants
d. Uncontrolled hypertension
8. Which dietary patterns are most consistently associated with general eye health?
a. Calorie-restricted high-protein diets
b. Vegetarian diets
c. Low-glycemic index diets
d. Mediterranean-style diets
9. What did the Age-Related Eye Disease Studies, AREDS and AREDS2, conclude about the use of a supplement with vitamins C and E, β-carotene, zinc, and copper?
a. The supplement reduces progression to late-stage AMD
b. The supplement prevents AMD
c. The supplement works better with Lu and Zx
d. The supplement has no effect on AMD
10. Which of the following best describes the goals for MNT for diabetic retinopathy?
a. Increased intake of dark green leafy vegetables and improved serum retinol levels
b. Increased protein intake and normalized retinol-binding protein levels
c. Good glycemic and hypertension control, and normalized lipid levels
d. A low-glycemic index diet and blood glucose monitoring
References
1. Lawrenson JG, Downie LE. Nutrition and eye health. Nutrients. 2019;11(9):2123.
2. Vision loss: a public health problem. Centers for Disease Control and Prevention website. https://www.cdc.gov/visionhealth/basic_information/vision_loss.htm. Updated June 12, 2020. Accessed February 15, 2022.
3. Teutsch SM, McCoy MA, Woodbury RB, Welp A, eds. Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington, D.C.: The National Academies Press; 2016.
4. Maia SB, Souza ASR, Caminha MFC. Vitamin A and pregnancy: a narrative review. Nutrients. 2019;11:681.
5. Otten JJ, Hellwig JP, Myers LD, eds. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C.: The National Academies Press; 2006:170-179.
6. McCauley ME, van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015;2015(10):CD008666.
7. Crum AR, Srikumaran D, Woreta F. Bitot’s spots following bariatric surgery: an ocular manifestation of a systemic disease. Case Rep Ophthalmol. 2017;8(3):581-589.
8. Demmig-Adams B, Adams RB. Eye nutrition in context: mechanisms, implementation, and future directions. Nutrients. 2013;5(7):2483-2501.
9. Addo EK, Gorusupudi A, Allman S, Bernstein PS. The lutein and zeaxanthin in pregnancy study – carotenoid supplementation during pregnancy: ocular and systemic effects – study protocol for a randomized controlled trial. Trials. 2021;22(1):300.
10. Abdel-Aal el-SM, Akhtar H, Zaheer K, Ali R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients. 2013;5(4):1169-1185.
11. Rasmussen HM, Johnson EJ. Nutrients for the aging eye. Clin Interv Aging. 2013;8:741-748.
12. Pellegrini M, Senni C, Bernabei F, et al. The role of nutrition and nutritional supplements in ocular surface diseases. Nutrients. 2020;12(4):952.
13. Ramdas WD. The relation between dietary intake and glaucoma: a systematic review. Acta Ophthalmol. 2018;96(6):550-556.
14. Omega-3 fatty acids. National Institutes of Health website. https://www.ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/. Updated August 4, 2021. Accessed May 17, 2022.
15. Gopinath B, Liew G, Kifley A, et al. Dietary flavonoids and the prevalence and 15-year incidence of age-related macular degeneration. Am J Clin Nutr. 2018;108(2):381-387.
16. Millen AE, Meyers KJ, Liu Z, et al. Association between vitamin D status and age-related macular degeneration by genetic risk. JAMA Ophthalmol. 2015;113(10):1171-1179.
17. Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-year incidence of age-related macular degeneration. Am J Clin Nutr. 2013;98(1):129-135.
18. Merle BM, Silver RE, Rosner B, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced non-exudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr. 2016;103(4):1135-1144.
19. Ramdas WD, Schouten JSAG, Webers CAB. The effect of vitamins on glaucoma: a systematic review and meta-analysis. Nutrients. 2018;10(3):359.
20. Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and mesozeaxanthin: the basic and clinical science underlying carotenoid-based, nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34-66.
21. Sun H, Cheng R, Wang Z. Early vitamin A supplementation improves the outcome of retinopathy of prematurity in extremely preterm infants. Retina. 2020;40(6):1176-1184.
22. Mun JG, Legette LL, Ikonte CJ Mitmesser SH. Choline and DHA in maternal and infant nutrition: synergistic implications in brain and eye health. Nutrients. 2019;11(5):1125.
23. Schneider N, Garcia-Rodenas CL. Early nutritional interventions for rain and cognitive development in preterm infants: a review of the literature. Nutrients. 2017;9(3):187.
24. Raghuveer TS, Bloom BT. A paradigm shift in the prevention of retinopathy of prematurity. Neonatology. 2011;100(2):116-129.
25. Bashinsky AL. Retinopathy of prematurity. N C Med J. 2017;78(2):124-128.
26. Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017;7(7):CD000254.
27. Brandão E, João HS. Genetics of AMD. GER Retina Study Group website. http://www.amdbook.org/content/genetics-amd. Published 2010. Accessed March 3, 2022.
28. Merle BM, Silver RE, Rosner B, Seddon JM. Adherence to a Mediterranean diet genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015;102(5):1196-1206.
29. Buscemi S, Corleo D, Di Pace F, Petroni ML, Satriano A, Marchesini G. The effect of lutein on eye and extra-eye health. Nutrients. 2018;10(9):1321.
30. Age-related eye disease studies (AREDS, AREDS2). National Eye Institute website. https://www.nei.nih.gov/research/clinical-trials/age-related-eye-disease-studies-aredsareds2. Updated April 13, 2020. Accessed March 29, 2022.
31. Taylor HR, Tikellis G, Robman LD, McCarty CA, McNeil JJ. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ. 2002;325(7354):11.
32. Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124(8):1151-1162.
33. Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686.
34. Glaucoma facts and stats. Glaucoma Research Foundation website. http://www.glaucoma.org/glaucoma/glaucoma-facts-and-stats.php Updated October 29, 2017. Accessed March 31, 2022.
35. Pasquale LR, Kang JH. Lifestyle, nutrition, and glaucoma. J Glaucoma. 2009;18(6):423-428.
36. Jung KI, Kim YC, Park CK. Dietary niacin and open-angle glaucoma: the Korean National Health and Nutrition Examination Survey. Nutrients. 2018;10(4):387.
37. Wang SY, Singh K, Lin SC. The association between glaucoma prevalence and supplementation with the oxidants iron and calcium. Invest Opthalmol Vis Sci. 2012;53(2):725-731.
38. Pérez de Arcelus M, Toledo E, Martínez-González MÁ, Sayón-Orea C, Gea A, Moreno-Montañés J. Omega 3:6 ratio intake and incidence of glaucoma: the SUN cohort. Clin Nutr. 2014;33(6):1041-1045.
39. Weikel KA, Garber C, Baburins A, Taylor A. Nutritional modulation of cataract. Nutr Rev. 2014;72(1):30-47.
40. Braakhuis AJ, Donaldson CI, Lim JC, Donaldson PJ. Nutritional strategies to prevent lens cataract: current status and future strategies. Nutrients. 2019;11(5):1186.
41. Lim JC, Caballero Arrendondo M, Braakhuis AJ, Donaldson PJ. Vitamin C and the lens: new insights into delaying the onset of cataract. Nutrients. 2020;12(10):3142.
42. McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract. Ophthalmology. 2004;111(1):75-84.
43. Li LH, Lee JC, Leung HH, Lam WC, Fu Z, Lo ACY. Lutein supplementation for eye diseases. Nutrients. 2020;12(6):1721.
44. Glaser TS, Doss LE, Shin G, et al. The associations of dietary lutein plus zeaxanthin and B vitamins with cataracts in the Age-Related Eye Disease Study: AREDS Report No. 37. Ophthalomology. 2015;122(7):1471-1479.
45. Dry eye. National Eye Institute website. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/dry-eye. Updated December 22, 2020. Accessed September 10, 2021.
46. Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22(4):279-282.
47. Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902-1912.
48. Wright AD, Dodson PM. Medical management of diabetic retinopathy: fenofibrate and ACCORD Eye studies. Eye (Lond). 2011;25(7):843-849.
49. Chous AP, Richer SP, Gerson JD, Kowluru RA. The Diabetes Visual Function Supplement Study (DiVFuSS). Br J Ophthalmol. 2016;100(2):227-234.
50. Hari T, Elsherbiny S. Bariatric surgery – what the ophthalmologist needs to know. Eye (Lond). 2022;36(6):1147-1153.
51. Francisco SG, Smith KM, Aragonès G, et al. Dietary patterns, carbohydrates, and age-related eye diseases. Nutrients. 2020;12(9):2862.
52. Whitehead M, Wickremasinghe S, Osborne A, Van Wijngaarden P, Martin KR. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opin Biol Ther. 2018;18(12):1257-1270.
53. Diabetic retinopathy. National Eye Institute website. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/diabetic-retinopathy. Updated March 25, 2022. Accessed April 3, 2022.
54. Moïse MM, Benjamin LM, Doris TM, Dalida KN, Augustin NO. Role of Mediterranean diet, tropical vegetables rich in antioxidants, and sunlight exposure in blindness, cataract, and glaucoma among African type 2 diabetics. Int J Ophthalmol. 2012;5(2):231-237.
55. Braakhuis A, Raman R, Vaghefi E. The association between dietary intake of antioxidants and ocular disease. Diseases. 2017;5(1):3.
56. Chiu CJ, Chang M, Zhang FF, et al. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol. 2014;158(1):118-127.e1.
57. Jones AM. Physician Knowledge, Attitudes, and Behaviors Towards Registered Dietitian Nutritionists. Doctoral dissertation. University of North Florida; 2021. https://digitalcommons.unf.edu/etd/1016.
