April 2018 Issue
April 2018 Issue
Soy Under Fire
By Matthew Ruscigno, MPH, RD
Today's Dietitian
Vol. 20, No. 4, P. 18
Today's Dietitian gives an overview of what led to soy's stellar health claim and why the FDA is considering downgrading the science.
There may not be a single food that's as maligned and political as the soybean. In a world of "all foods fit," soy is a battleground food, one where the sides aren't clearly defined and science-based arguments are thrown out the window faster than one can say "estrogenlike compounds." Some health food advocates are firmly against its consumption, claiming it's a cancer-causing product of biotech companies, while proindustry stand-with-science adherents dismiss it as an overly hyped, fraudulent food with a health halo it doesn't deserve.
The history of soy is long and fascinating, worthy of its own documentary. It's a legume, but unlike most, save the peanut, it has spawned an incredible variety of items including milks, ice creams, miso, hamburgers, hot dogs, oil, soy sauce, natto, tempeh, a vast array of tofus, and more. In addition, many studies have suggested a relationship between soy protein and lower risk of heart disease.
There's an abundance of research looking at health outcomes related to consumption of soyfoods, yet the discussion primarily revolves around opinions, fear, and posturing based on which camp one happens to be in.
The FDA is presently revisiting its position on soy; changes to its official status may be in store. This article will familiarize RDs with the particulars so they're prepared to offer guidance when counseling clients.
FDA-Authorized Health Claim
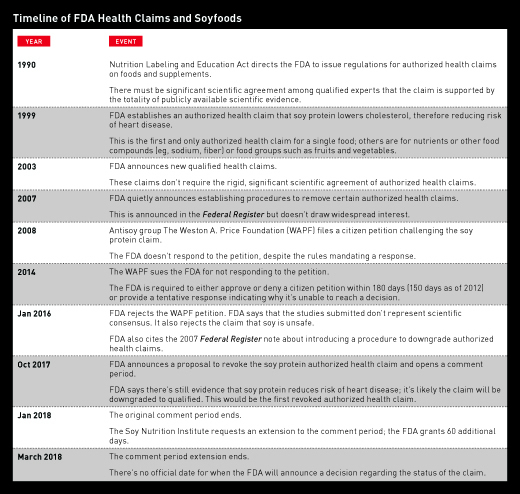
The Nutrition Labeling and Education Act of 1990 amended the Federal Food, Drug, and Cosmetic Act regarding how the FDA regulates health claims on food labels. This allowed the FDA to develop what are formally called authorized health claims, which can be applied to a specific nutrient or food and a related health outcome. These claims, according to the FDA, require "significant scientific agreement," and only 12 have been approved in the nearly 30 years since authorization.1
The two official versions of the soy protein and heart disease risk authorized health claims, as approved by the FDA since 1999, are as follows:
• Twenty-five grams soy protein per day, as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease. A serving of [name of food] supplies __ g of soy protein.
• Diets low in saturated fat and cholesterol that include 25 g soy protein per day may reduce the risk of heart disease. One serving of [name of food] provides __ g soy protein.2
Examples of other health claims that are well known in the nutrition field and are somewhat less controversial include that certain fruits and vegetables may reduce cancer risk, and that fiber consumption may lower coronary heart disease (CHD) risk.
Significant scientific agreement is no small accomplishment, as demonstrated by the small number of authorized health claims. The quantity and quality of research needed to earn this statement requires a plethora of studies and in some cases more than a decade of review and evaluation. The original authorized health claim for soy is explained in a 34-page document with 167 references that demonstrate an agreement among the scientific community that soy protein consumption lowers total and LDL serum cholesterol. The claim is based on the accepted premise that lowering total and LDL cholesterol levels reduces heart disease risk.2 Of all the authorized health claims, soy is the only specific food; the rest include nutrients or food components such as fiber, calcium, or sodium or food groups/categories such as fruits and vegetables.
The FDA, however, has now proposed a rule to revoke soy's authorized health claim due to inconsistent study findings on the link between soy and heart health. Should the FDA finalize the rule, it will allow the use of a qualified health claim if there's sufficient evidence supporting an association between eating soy protein and a lower risk of heart disease. In the history of FDA authorized health claims, no claim has ever been revoked, but it's unique that such a claim for a specific foodstuff exists in the first place.
The Case Against Soy
Despite soy's popularity, a movement mounted against its esteemed reputation. One group at the forefront of the antisoy crusade is The Weston A. Price Foundation (WAPF). Founded in 1999 by Sally Fallon Morell and Mary G. Enig, who holds a PhD in nutrition, the group's philosophy is based on the teachings of Weston A. Price, a dentist. They advocate for a return to a wholesome farm diet rich in beef, eggs, and full-fat dairy products. In August 2008, The WAPF filed a citizen petition challenging the FDA's final rule that allows health claims to be made about the effect of soy protein on CHD. The FDA is required to approve or deny such petitions, or provide a tentative response explaining why it hasn't reached a decision. For unknown reasons, the FDA didn't respond to the petition in a timely fashion. In 2014, The WAPF filed a lawsuit to compel the FDA to provide a substantive response to the 2008 petition.3
Finally, in 2016, the FDA responded directly to the WAPF petition. Interestingly, the FDA claims that in 2007 it stated, via the Federal Register, that it would be reevaluating the scientific evidence for soy protein and risk of CHD and intended to create a new rule for revoking authorized health claims. It's unclear why the FDA waited eight years to respond if it was already planning to reevaluate the evidence.4
Despite the ongoing reevaluation, the FDA rejected much of The WAPF citizen petition: "As discussed, no scientific conclusions could be drawn from the vast majority of publications you cited. The six relevant studies you cited did not show a benefit for intake of soy protein and risk reduction of CHD. Although FDA considers these studies to be relevant to its reevaluation of the soy protein and CHD health claim, they do not represent the totality of publicly available scientific evidence on soy protein and risk reduction of CHD."4
The FDA also rejected The WAPF's claims in the petition that soy isn't qualified for any health claim because it's unsafe for consumption and associated with negative health outcomes.4 The WAPF had argued, in a different lawsuit against the state of Illinois, that high soy consumption causes painful constipation, debilitating diarrhea, vomiting, heart palpitations, rashes, acne, insomnia, panic attacks, brain fog, fatigue, weight gain, and thyroid disease.5
In October 2017, the FDA released an official document explaining the proposed removal of the authorized health claim, citing new research that had come out since the designation of the claim in 1999.6 Mark Messina, PhD, executive director of the Soy Nutrition Institute, says that many of the studies the FDA cited were underpowered, which is why there wasn't a strong association between soy consumption and reduction in LDL cholesterol. "Health Canada looked not just at statistical significance but [also] the directionality of the data. They found 80% of the studies showed a reduction although only 33% were statistically significant."7 In 2015, Health Canada established an official health claim that's similar to the FDA authorized health claim regarding the ability of soy protein to lower total and LDL cholesterol and reduce heart disease risk. Health Canada has no plans to reevaluate their 2015 claim at this time.
Downgrading to a Qualified Health Claim
In October 2017, the FDA released an official statement on the proposal to revoke soy protein's authorized health claim: "While some evidence continues to suggest a relationship between soy protein and a reduced risk of heart disease—including evidence reviewed by the FDA when the claim was authorized—the totality of currently available scientific evidence calls into question the certainty of this relationship. For example, some studies, published after the FDA authorized the health claim, show inconsistent findings concerning the ability of soy protein to lower heart-damaging LDL cholesterol."8
Through the Consumer Health Information for Better Nutrition Initiative of 2003, the FDA acknowledged that the general population benefits from having more nutrition and health information on packaging. As part of this initiative, the agency established procedures so qualified health claims can be made for foods in addition to supplements (the Dietary Supplement Health and Education Act of 1994 already had established some special regulatory requirements and procedures for using structure/function claims on supplements).
According to FDA documents, qualified health claims differ from significant scientific agreement health claims, or authorized health claims, in that they must be accompanied by a disclaimer or otherwise qualified. In other words, qualified health claims don't have the level of evidence required to be an authorized health claim, but enough evidence exists to make a qualified claim.9 The FDA is arguing that this is the case for soy's association with lower cholesterol levels since the current research is weaker now than it was in 1999. The question comes down to this: Is the evidence on the reduction of total and LDL cholesterol levels from soy protein consumption strong enough to glean significant scientific agreement? Health Canada believes so, as do the scientists of the Soy Nutrition Institute, but in the end it's up to the FDA.
The FDA extended the comment period on whether soy protein should continue to have an authorized health claim until March 19, 2018. The Soy Nutrition Institute submitted comments and relevant research in support of maintaining the authorized health claim. It appears likely that the soy health claim will be downgraded from authorized to qualified, though no one can say for sure until the FDA issues its final rule. The date of the final rule is unknown.
Should Dietitians Recommend Soy to Lower Cholesterol and CHD Risk?
As someone who's closely followed the issues surrounding soy from the beginning, my professional position has adjusted as the field has changed over the last two decades. The nutrition field is moving away from promoting the benefits of singular foods and looking at overall dietary patterns. Focusing on isolated foods and nutrients has lead to soy protein-fortified potato chips and fiber-enriched, sugary breakfast cereals, which are not ideal for providing health benefits or preventing heart disease.
Your average clients who are at risk of CVD would most likely benefit from adding soyfoods to their diets. The more difficult questions to answer are: Is the reduction in total and LDL cholesterol due to the fiber, the plant protein, or the phytochemicals? Or, is the reduction in total and LDL cholesterol from soy greater than it would be from the addition of any other legume?10 Of course, the overall dietary pattern matters most, and it's unlikely that the only change one would make is to add soy protein foods. It's important to remember that nowhere in this discussion does the FDA question whether soy protein lowers cholesterol. The concern is by how much. Combined with other cholesterol-reducing dietary changes, soy protein can play an important role in reducing heart disease risk. Soy products are much more common than they were in 1999 and are readily available in versions of milks and meats that are recognizable to the average consumer.
— Matthew Ruscigno, MPH, RD, is chief nutrition officer at Nutrinic, a health care company promoting the use of plant-based diets in the prevention of CVD.
References
1. Guidance for industry: FDA's implementation of "qualified health claims": questions and answers; final guidance. Food and Drug Administration website. https://www.fda.gov/RegulatoryInformation/Guidances/ucm053843.htm. Updated November 8, 2017. Accessed February 4, 2018.
2. Department of Health and Human Services, Food and Drug Administration. Food labeling: health claims; soy protein and coronary heart disease. https://www.gpo.gov/fdsys/pkg/FR-1999-10-26/pdf/99-27693.pdf. Published October 26, 1999. Accessed February 4, 2018.
3. Hartke K. Nutrition watchdog files lawsuit against FDA. The Weston A. Price Foundation website. https://www.westonaprice.org/soy-lawsuit-filed. Published December 17, 2014. Accessed February 8, 2018.
4. Citizen petition denial response letter from FDA CFSAN to The Weston A. Price Foundation. Regulations.gov website. https://www.regulations.gov/document?D=FDA-2008-P-0452-0011. Published January 20, 2016. Accessed March 4, 2018.
5. Experts denounce high soy diet of Illinois prisoners. The Weston A. Price Foundation website. https://www.westonaprice.org/experts-denounce-high-soy-diet-of-illinois-prisoners-2. Published June 26, 2012. Accessed February 8, 2018.
6. Food labeling: health claims; soy protein and coronary heart disease. Federal Register website. https://www.federalregister.gov/documents/2017/10/31/2017-23629/food-labeling-health-claims-soy-protein-and-coronary-heart-disease. Published October 31, 2017. Accessed February 4, 2018.
7. Summary of Health Canada's assessment of a health claim about soy protein and cholesterol lowering. Government of Canada website. https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/summary-assessment-health-claim-about-protein-cholesterol-lowering.html. Updated March 18, 2015. Accessed March 4, 2018.
8. Statement from Susan Mayne, Ph.D., on proposal to revoke health claim that soy protein reduces risk of heart disease. Food and Drug Administration website. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm582744.htm. Updated February 23, 2018.
9. Label claims for conventional foods and dietary supplements. Food and Drug Administration website. https://www.fda.gov/Food/LabelingNutrition/ucm111447.htm. Updated January 3, 2018. Accessed February 8, 2018.
10. Li SS, Blanco Mejia S, Lytvyn L, et al. Effect of plant protein on blood lipids: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2017;6(12):e006659.
[Sidebar 1]
UNDERSTANDING FDA HEALTH CLAIMS
A "health claim" by definition has two essential components: 1) a substance, whether a food, food component, or dietary ingredient; and 2) a disease or health-related condition. A statement lacking either one of these components doesn't meet the regulatory definition of a health claim. For example, statements that address a role of dietary patterns or of general food categories (eg, fruits and vegetables) in maintaining good health are considered to be dietary guidance rather than health claims.
Dietary guidance statements used on food labels must be truthful and not misleading. Statements that address a role of a specific substance in maintaining normal healthy structures or functions of the body are considered to be structure/function claims.
Unlike health claims, dietary guidance statements and structure/function claims aren't subject to premarket review and FDA authorization.
— Source: FDA
[Sidebar 2]
DISPELLING COMMON SOY MYTHS
Since 1990, there have been more than 10,000 peer-reviewed research studies done on soy and its compounds. We can say with certainty that soy is safe and that it's beneficial to overall health. Here are some of the most common myths about soy and the facts that dispel them.
Myth #1: Soy contains estrogen.
Fact: The benefit of soy and one of the main causes for criticism is the phytochemical group called isoflavones. Phytochemicals are compounds in plants that have nonnutritive benefits and are studied extensively for their role in chronic disease prevention.1 Isoflavones have been called phytoestrogens because they bind to some estrogen receptors in the body, but their activity isn't the same as estrogen; in some cases they have antiestrogenic effects.
There were reports of feminization of men due to soy, but this was at a level of more than 14 servings per day. Studies that examined up to six servings per day haven't shown similar results nor has this consumption level negatively affected sperm count.2
Myth #2: Soy contains antinutrients.
Fact: Soy, like other plant foods, contains phytates that could theoretically reduce the absorption of some minerals. In practice, there's no evidence to support increased risk of vitamin or mineral deficiencies in those who consume soy.3
The calcium in soymilk is absorbed at about the same rate as that from cow's milk and ferritin, the type of iron found in large amounts in soy. And the calcium is absorbed particularly well by those with low iron stores.4 Firm tofu is an especially good source of nonheme iron and adding a vitamin C-containing food significantly increases absorption.5
Myth #3: Only fermented soyfoods are health promoting.
Fact: Some argue that soy is only a condiment in most Asian cultures or that the majority of soy consumed is fermented, but the evidence doesn't support these claims.
In Japan, fermented forms are regularly consumed, as is tofu. Most of the soy consumed in China isn't fermented, and in Indonesia, where tempeh is a common food, nonfermented types of soy also are eaten.6 The number of phytochemicals in fermented vs nonfermented soyfoods varies little. Extensive research on cancer says that soy is neutral or protective for many types including breast and prostate.7
— MR
References
1. Messina M, Watanabe S, Setchell KD. Report on the 8th International Symposium on the Role of Soy in Health Promotion and Chronic Disease Prevention and Treatment. J Nutr. 2009;139(4):796S-802S.
2. Beaton LK, McVeigh BL, Dillingham BL, Lampe JW, Duncan AM. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men. Fertil Steril. 2010;94(5):1717-1722.
3. Lopez HW, Leenhardt F, Coudray C, Remesy C. Minerals and phytic acid interactions: is it a real problem for human nutrition? Int J Food Sci Technol. 2002;37(7):727-739.
4. Zhao XF, Hao LY, Yin SA, Kastenmayor P, Barclay D. A study on absorption and utilization of calcium, iron and zinc in mineral-fortified and dephytinized soy milk powder consumed by boys aged 12 to 14 years. Zhonghua Yu Fang Yi Xue Za Zhi. 2003;37(1):5-8.
5. Murray-Kolb LE, Welch R, Theil EC, Beard JL. Women with low iron stores absorb iron from soybeans. Am J Clin Nutr. 2003;77(1):180-184.
6. Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1-12
7. Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89(4):1155-1163.