Today’s Dietitian
Vol. 19, No. 2, P. 36
Here’s a review of what the new guidance around “healthy” labeling means for dietitians and consumers.
Since 1993, the FDA’s definition of the implied nutrient content claim “healthy” hasn’t changed, but what has changed is the evidence surrounding total fat intake and nutrients of public health importance.
This prompted the FDA to issue guidance in September 2016 allowing manufacturers some latitude when making the claim “healthy” on product labels. “As our understanding of nutrition science evolves, we need to make sure the definition for the ‘healthy’ nutrient content claim remains up to date,” says Douglas Balentine, PhD, director of the office of nutrition and food labeling at the FDA in Washington, D.C. “For instance, the most recent public health recommendations now focus on type of fat rather than amount of fat. The guidance announces that in certain circumstances, FDA intends not to enforce the labeling requirements for companies that use the term ‘healthy’ as a nutrient content claim while we work on revising the definition.”
Under the new guidance, manufacturers are now allowed to label products as “healthy” if they meet any of the following criteria in addition to other criteria for the claim: Their products aren’t low in total fat but have a fat composition of mostly monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids, and include the amounts of MUFAs and PUFAs on the Nutrition Facts label; or they contain at least 10% DV per reference amount customarily consumed (RACC) for potassium or vitamin D and include these amounts on the Nutrition Facts label.1 However, it’s important to note that this is only a guidance document and not a change made in the regulation or actual definition of “healthy” as the FDA sees it. The FDA is soliciting public comments on what should be the new definition of “healthy” until April 26, 2017. After this date, FDA will review all the comments to help determine the new definition.2
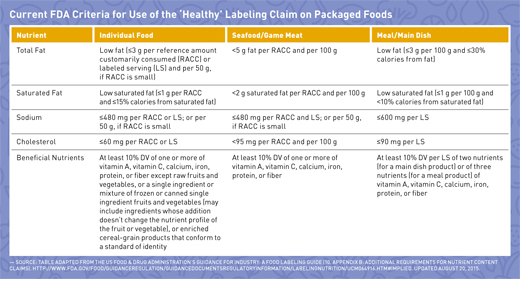
When the term “healthy” or any similar term, such as “healthful,” “healthier,” or “healthiest,” is used on food labels, the food must have fewer amounts of certain nutrients to limit, including total fat, saturated fat, cholesterol, and sodium, and contain at least 10% of certain nutrients to encourage in one labeled serving or RACC (See table on page 38). “Many people may be under the assumption that serving sizes are a recommendation. They are, in fact, a reference based on what people typically consume, not what should be consumed,” says Kris Sollid, RD, director of nutrition communications at the International Food Information Council (IFIC) in Washington, D.C., a nonprofit organization whose members include food industry companies. This is an important reminder for dietitians when counseling clients on using the serving size information on food labels.
RDs may be asking themselves why sugar isn’t included in the list of criteria. The answer is mainly that while keeping sugar intake low always has been recommended in the Dietary Guidelines for Americans, it has become more of a focal point today than it was when the original definition of “healthy” was developed in the 1990s.3 Unfortunately, this led to a definition of “healthy” that can be labeled on fat-free pudding and sugar-laden cereal but not on nuts, avocados, or salmon. Manufacturers have responded by asking the FDA to update nutrient content claim regulations for consistency with current dietary recommendations.
A recent citizen petition submitted by snack maker KIND stated that the existing regulation “limits the ability of food producers to tell consumers that products containing certain foods—such as nuts, whole grains, seafood, fruits, and vegetables—are healthy, even though they’re currently recommended as key components of a healthful diet.”4
According to Stephanie Perruzza, MS, RD, CDN, health & wellness specialist at KIND Healthy Snacks in New York City, “In addition to prizing real food and wholesome ingredients, we think the definition of ‘healthy’ should be consistent with modern nutrition science. We plan to reiterate this stance when submitting our public comments as part of the FDA’s open comment period.”
Why Now?
In July 2016, the FDA released a strategic plan outlining four goals, one being nutrition, which includes “providing and supporting accurate and useful nutrition information to consumers so they can choose more healthful diets consistent with the Dietary Guidelines for Americans and other evidence-based recommendations; and encouraging and facilitating new products and product reformulation to promote a healthier food supply.” The FDA already has begun supporting this objective by releasing revisions to the Nutrition Facts label and serving size information for packaged foods to reflect new scientific information.5,6 Because the backbone of many of the FDA’s nutrient content claim regulations is based on Nutrition Facts label and serving size regulations, the FDA is updating these regulations to align with one another, beginning with the regulation around the definition of “healthy.” The original definition of “healthy” in 1993 was meant to imply that the nutrient content of a food could contribute to a diet consistent with dietary intake recommendations. The most current Dietary Guidelines for Americans at that time, the 1990 edition, recommended eating a diet low in fat, saturated fat, and cholesterol. However, the science and recommendations related to fat intake have shifted and no longer focus on total fat intake overall. In fact, the most current Dietary Guidelines for Americans, the 2015–2020 edition, recommend consumers replace saturated fats with unsaturated fats, primarily PUFAs and MUFAs, and suggest that it’s consumers’ eating patterns overall that contribute to a healthful diet and not necessarily nutrients eaten in isolation.7 Since the current regulation is antiquated in requiring that a food be low fat to be labeled “healthy,” the FDA is now allowing the “healthy” claim on foods that aren’t low fat but have a fat profile of predominantly MUFAs and PUFAs if manufacturers also declare the amounts of these fatty acids on the label.
The current definition of “healthy” also focuses on foods providing at least a good source of nutrients that historically have been nutrients of public health concern, including vitamin A, vitamin C, iron, calcium, protein, and dietary fiber. However, eating patterns have shifted over the years, and potassium and vitamin D have replaced vitamins A and C as nutrients of public health concern.7 This shift already has been reflected in a change to the mandatory nutrients that must be stated on the Nutrition Facts label, and now the FDA is exercising enforcement discretion for “healthy” labeling to allow manufacturers to consider foods containing at least 10% DV per RACC of potassium and vitamin D.
Considerations for Dietitians
Based on public comments, the definition of “healthy” could be completely different from what we’ve seen historically. The FDA is asking for public comment on whether “healthy” should be based only on nutrient content, whether all food categories should be subject to the same criteria, whether there are other words or terms that may be more appropriate (eg, “nutritious”), and what are consumers’ expectations of foods carrying a “healthy” claim. While the FDA’s current definition of “healthy” refers to a set of nutrient content criteria, what constitutes a healthy food varies among dietitians as well as consumers. Survey data from the IFIC Foundation indicate people define a “healthy” food in different ways, with about one-third of people reporting they look for what a food doesn’t contain, not what it does contain, when considering whether it’s healthy.8 These findings parallel many of the public comments FDA has received regarding what should be the new definition of “healthy,” with consumers writing in saying that “healthy” should be allowed only on foods with no “artificial ingredients” or “GMOs.” Commenters also have stated that nutrient-based labeling claims are misleading and that claims on the food label should instead be based on overall eating patterns.
In the short term, nutrition professionals may not see many changes to actual food labels based on the FDA’s new guidance on use of the word “healthy” on packaged foods. Many manufacturers say it’s too early in the process to make any changes to their labels, and that they’ll be waiting on more formal regulations. However, if manufacturers choose to label packaged foods that aren’t low fat but mostly contain PUFAs and MUFAs as “healthy,” then consumers will be able to find these beneficial fatty acids on some food labels. (MUFAs and PUFAs are voluntary nutrients, so typically they aren’t included on most Nutrition Facts labels.)
As with anything food related, dietitians can play an important role in helping clients sift through the changing and sometimes confusing nutrition labeling information. For example, while ready-to-eat food items bearing a “healthy” label sold in grocery stores (think packaged salads and deli sandwiches) must comply with the definition, the FDA’s guidance around “healthy” and other regulations on food labeling don’t extend to grocery stores’ programming or branding themselves. This means that internal retail programs such as Aldi’s Healthier Checklanes initiative and the Houston-based Healthy Checkout Aisle program can have whatever criteria they desire, or none, creating a dilemma for supermarket RDs especially when trying to communicate what “healthy” means for their products, their stores, and their customers.
Dietitians like Megan Ware, RDN, LD, owner of Nutrition Awareness in Orlando, Florida, are looking beyond the front of the food package to educate clients about what really makes a food healthful. “I do not encourage clients to look for the word ‘healthy’ on labels. I do encourage clients to skip past the marketing claims, photos, and endorsements on any product and go straight to the ingredient label. What’s healthy for one person may not be for the next depending on their specific health condition and situations, so I do not think labeling products as ‘healthy’ is necessarily beneficial,” Ware says.
Sollid adds that “just because a food can’t be labeled as ‘healthy’ doesn’t mean it wouldn’t fit into a ‘healthy diet,’ and this is where the supermarket dietitian comes in.”
According to Annette Maggi, MS, RDN, LD, FAND, president of Annette Maggi & Associates, Inc, a food marketing and communications company whose clients include food companies, grocery retailers, and related industries in the greater Minneapolis-St. Paul area, “While individual foods may be labeled ‘healthy,’ this doesn’t necessarily translate to overall healthy eating habits. For this reason, it’s exciting to see the continued expansion of dietitians’ roles in the retail grocery industry. These dietitians can guide consumers at the point of purchase to build healthful meal patterns and eating habits, looking beyond the nutrition profile of individual foods.”
Other FDA Updates
In addition to the new guidance on the term “healthy,” the FDA is addressing the need to update other labeling claims such as “low sodium” and “good source” to bring them in line with current public health recommendations and new requirements for the Nutrition Facts label. For example, current labeling regulations require packaged foods not considered low in total fat to disclose total fat content per labeled serving if making a claim about fiber content—an outdated requirement in light of the new recommendations on fat intake. Furthermore, far fewer foods will qualify for “good source” claims since the DV for nearly all nutrients have increased. Foods bearing a “good source” claim must contain at least 10% DV of the nutrient per RACC.
As far as a timeline for when dietitians may see the new definition of “healthy,” don’t expect it any time soon. According to the FDA, the information submitted in response to the Federal Register notice will be used to develop a proposed rule, which also will be published for public comment. After that, the FDA again will consider the submitted comments to help inform and develop a final rule. The whole process could take years. “The amount of time rulemaking takes depends on many factors, and as a result it’s too early in the process to identify when a new, final definition of ‘healthy’ will be in effect,” Balentine says.
In the meantime, what does the FDA recommend dietitians do when speaking with clients about choosing healthful foods? “While nutrient content claims like ‘healthy’ can be a helpful tool for consumers, the best way to assess whether a product fits into a healthful diet is to look at the Nutrition Facts label,” Balentine says. “Read the nutrient contents and the ingredients list to make an informed determination about whether a given product is part of a healthful, balanced diet. In addition, dietitians may want to look for substantiated health claims that also can provide information about the role of these foods in healthful diet patterns.”
As Ware and Sollid recommend, dietitians should instruct clients to look beyond the label when deciphering the healthfulness of a food. Front-of-package label claims such as “healthy” are tools to help consumers more easily identify better-for-you foods but shouldn’t be the only resource for gauging what’s healthful and what’s not. When counseling clients about eating healthful foods, RDs should consider what “healthy” means in the context of their clients’ overall eating patterns, and remember to not judge a food solely by its label.
— Jessica Levings, MS, RDN, is a freelance writer and owner of Balanced Pantry, a consulting business helping companies develop and modify food labels, conduct recipe analysis, and create nutrition communications materials. Learn more at www.balancedpantry.com, Twitter @balancedpantry, and Facebook.com/BalancedPantry1.
References
1. US Food and Drug Administration. Use of the term “healthy” in the labeling of human food products: guidance for industry: http://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/UCM521692.pdf. Published September 2016.
2. Use of the term ‘healthy’ in the labeling of human food products. Regulations.gov website. https://www.regulations.gov/docket?D=FDA-2016-D-2335
3. US Department of Health and Human Services, US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans: Eighth Edition. http://health.gov/dietaryguidelines/2015/guidelines/. Published January 7, 2016.
4. KIND LLC. Citizen petition. https://s3.amazonaws.com/kind-docs/citizen-petition.pdf. Published December 1, 2015.
5. Food labeling: revision of the nutrition and supplement facts label. Regulations.gov website. https://www.regulations.gov/document?D=FDA-2012-N-1210-0875. Published May 27, 2016.
6. Food labeling: serving sizes of foods that can reasonably be consumed at one eating occasion; dual-column labeling; updating, modifying, and establishing certain reference amounts customarily consumed; serving size for breath mints; and technical amendments. Regulations.gov website. https://www.regulations.gov/document?D=FDA-2004-N-0258-0136. Published May 27, 2016.
7. US Department of Agriculture, Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 1990. http://origin.www.cnpp.usda.gov/Publications/DietaryGuidelines/1990/1990CommitteeReport.pdf
8. 2016 Food and Health Survey: food decision 2016: the impact of a growing national food dialogue. International Food Information Council Foundation website. http://www.foodinsight.org/articles/2016-food-and-health-survey-food-decision-2016-impact-growing-national-food-dialogue. Updated October 14, 2016.


