 January 2016 Issue
January 2016 Issue
CPE Monthly: Leaky Gut Syndrome — Learn About the Causes, Associated Conditions, and Treatments Under Research
By E.A. Stewart, MBA, RD
Today's Dietitian
Vol. 18 No. 1 P. 46
Suggested CDR Learning Codes: 5120, 5220
Suggested CDR Performance Indicators: 8.1.3, 8.3.6, 10.4.4
CPE Level: 3
Take this course and earn 2 CEUs on our Continuing Education Learning Library
Leaky gut syndrome, defined as an "increase in intestinal permeability" by Peter Green, MD, director of the Celiac Disease Center at Columbia University,1 remains an enigma to most medical professionals, as much is still not known about this medical mystery. The intestine is lined with a thin but effective layer of cells that release mucus to protect the gut and allow transport of small molecules (eg, amino acids, electrolytes, water, and other nutrients) into the bloodstream to be used by the body. When this layer of cells is disrupted or inflamed, as occurs in celiac disease, larger molecules that would normally be blocked can enter the bloodstream.2
According to Daniel Leffler, MD, MA, director of clinical research at the Celiac Center at Beth Israel Deaconess Medical Center in Boston, leaky gut syndrome, or increased intestinal permeability, occurs when the complex of proteins known as "tight junctions" that link the cells lining the intestine are not working properly; this may allow proteins and microorganisms to get through the intestinal lining,3 causing disease or symptoms such as fatigue, gas, bloating, joint and muscle aches and pains, skin rashes, and confusion.
All of these symptoms are extremely variable, however, and can occur with many different diseases, making leaky gut syndrome all the more difficult to diagnose. Indeed, most of the published research refers to specific medical conditions potentially associated with leaky gut syndrome, as opposed to specific symptoms as mentioned above. In addition, some diseases, including celiac disease and irritable bowel syndrome (IBS), can affect intestinal permeability, making it impossible to separate cause from effect.4
Historically, most physicians have overlooked leaky gut, as it's not a diagnosis that's taught in medical school. Recently, however, more physicians and clinicians, including both conventional and alternative providers, are acknowledging that leaky gut syndrome is a real diagnosis and condition that needs further research and exploration because it has been linked to numerous medical conditions, including celiac disease, asthma, Crohn's disease, and cancer.
Alessio Fasano, MD, director of the Center for Celiac Research at Massachusetts General Hospital and noted researcher in the area of leaky gut syndrome, has suggested one reason the medical establishment remains skeptical about the existence of leaky gut syndrome is due to "ridiculous claims" made by some alternative medicine practitioners that "all diseases of humankind are due to leaky gut."5
To help dietitians and clinicians better understand leaky gut syndrome and the potential nutrition, pharmaceutical, and lifestyle strategies that may be used when working with patients affected by it, this continuing education course provides a broad overview of the literature that exists on leaky gut syndrome or intestinal permeability, including diagnosis, potential causes, medical conditions associated with the condition, and potential treatments.
Diagnosing Leaky Gut Syndrome
Few tests are available to determine if someone has leaky gut syndrome; however, one of the most commonly used tests to monitor intestinal permeability is the lactulose-mannitol challenge. While used mostly in clinical research, it's available in some commercial labs and may be a useful tool to track the efficacy of dietary and lifestyle changes on intestinal permeability over time.6
In this test, the patient swallows a solution of lactulose and mannitol, two metabolically inert sugars. Urine is then collected from the patient for six hours to assess for recovery of both sugars. In a healthy intestine, the mean absorption of mannitol, a monosaccharide, is 14% of the administered dose, whereas the mean absorption of lactulose, a disaccharide, is less than 1%. The normal ratio of lactulose-mannitol recovered in the urine is less than 0.03%, with an elevated ratio indicating intestinal hyperpermeability.7 Although the lactulose-mannitol challenge is commonly used in the research setting, interpretive problems may occur. In addition, variability among patients may occur due to their hydration status. Geographical location also may influence results, as a significant trend toward increased permeability in tropical locales has been noted.8 In addition, other factors, including the size of the patient and the duration of the urine collection, may alter the results of the test.9
Another test sometimes used in research settings to measure intestinal permeability is the 51-chromium-labeled ethylenediaminetetraacetic acid (51-Cr-EDTA) permeability test.10 With the 51-Cr-EDTA test, the patient ingests radio-labeled EDTA and the percentage of the chemical excreted in urine is monitored over a set time interval as determined by a gamma camera—a camera that detects radiation from a radioactive tracer injected into the body. As with the lactulose-mannitol challenge, day-to-day variability in intestinal permeability has been demonstrated, and use of the 51-Cr-EDTA test isn't recommended outside of the research setting.10
Zonulin and Increased Intestinal Permeability
Much of the research on leaky gut syndrome is focused on zonulin, a human protein that, to date, is the only physiological mediator known to regulate intestinal permeability reversibly by modulating intercellular tight junctions.11
Zonulin, which has been identified by Fasano and his colleagues as Pre-Haptaglobin-2, has been observed to be involved in intestinal innate immunity and to be upregulated in several autoimmune diseases, including celiac disease and type 1 diabetes.12,13 Two of the most powerful triggers of zonulin release include exposure to enteric bacteria (eg, Salmonella) and gluten, via gliadin, a glycoprotein present in wheat, in the small intestine.13
According to Fasano, zonulin works like the gatekeeper of the body's tissues, opening the spaces between cells and allowing some substances to pass through while keeping harmful bacteria and toxins out.14 It's thought that zolulin upregulation may precede the onset of type 1 diabetes, as one study detected elevated zonulin levels in 70% of subjects who went on to develop type 1 diabetes. In those subjects, elevated zonulin levels occurred 3.5 +/- 0.9 years before the onset of disease.15
Other Contributing Factors
Alcohol
Alcohol exposure can promote the growth of gram-negative bacteria in the intestine, which in turn can lead to the accumulation of acetaldehyde and a subsequential increase in intestinal permeability to endotoxin, a heat-stable toxin released by certain gram-negative bacteria upon cell disruption. In addition, alcohol-induced generation of nitric oxide also may lead to increased intestinal permeability and the subsequent disruption of intestinal barrier function.16
In one study of 36 alcoholic patients without liver cirrhosis or overt clinical evidence of malabsorption and malnutrition, intestinal permeability was measured via a 51-Cr-EDTA test. Patients who had abstained from alcohol for less than four days almost invariably had higher intestinal permeability than controls, and many patients continued to have abnormally higher levels for up to two weeks after cessation of drinking.17
Radiation and Chemotherapy
Chemotherapy and radiation have both been shown to affect intestinal barrier function and may lead to symptoms including abdominal pain, diarrhea, and bacterial infection.18 One small study of 10 patients with chemotherapy-induced stomatitis, the mucositis of oral mucosa, and 21 control cancer patients found evidence of increased intestinal permeability in those with stomatitis, as determined by measuring urinary lactulose, D-xylose, and mannitol. After treatment with oral granulocyte-monocyte colony-stimulating factor, lactulose excretion, lactulose-mannitol, and lactulose-xylose ratios decreased significantly, and symptoms of stomatitis improved in eight out of 10 patients.19
In another study, this one examining the effect of radiation therapy on intestinal permeability, segments from the sigmoid colon and rectum were obtained from irradiated and radiated patients. Intestinal permeability was measured via markers of C-mannitol, fluorescein isothiocyanate-dextran 4,400, and ovaalbumin, and passages of all markers were increased in the irradiated rectum compared with nonirradiated sigmoid colon, suggesting that gastrointestinal symptoms after radiation therapy may result from a loss of barrier integrity.18
NSAIDs
NSAIDs, such as indomethacin, naproxen, and ibuprofen,20 have been linked to increased intestinal permeability both with short-term use (within a 24-hour period) and long-term use (over a six-month period). One small study looked at patients with active rheumatoid arthritis, along with controls, and found that 86% of the patients not taking any prescribed NSAIDs had normal results, whereas 79% of patients taking NSAIDs had increased intestinal permeability. It wasn't determined, however, whether this increased permeability was due to the disease process, or due to NSAID therapy.21
Another study involving 68 patients who already had been taking one of six different NSAIDs for more than six months found evidence of intestinal inflammation in patients over the long term, with more than twice as many patients developing inflammation after six months vs three months. All types of conventional NSAIDs, except aspirin and nabumetone, were equally associated with small intestinal inflammation. Dosages were different for each type of NSAID, including daily ibuprofen dosages ranging from 1,200 to 2,400 mg per day and daily dosages of naproxen ranging from 1,000 to 1,500 mg per day.20
In addition, a review of studies that looked at the effect of NSAIDs on increased intestinal permeability revealed that virtually all studies agreed that conventional NSAIDs, as noted above, increase intestinal permeability in humans within 24 hours of ingestion and that this increased permeability also is apparent when NSAIDs are taken for the long term.21
Infectious Organisms
Many bacteria alter tight junction status, presumably to enhance their own growth requirements. Vibrio cholerae, for instance, secretes a variety of toxins including zonula occludens, which has been recognized as increasing paracellular permeability.22 In addition, increased intestinal permeability also may occur as the result of viral or parasitic infections, as evidenced by a study of 15 infants with rotavirus or Cryptosporidium infections.
In this study, an oral lactulose mannitol solution was administered to infants with rotavirus or Cryptosporidium and controls who were experiencing acute secretory diarrhea. Significant reductions in the mean lactulose-mannitol excretion ratios were observed between days one and 20 in children infected with rotavirus or Cryptosporidium, suggesting intestinal permeability caused by rotavirus or Cryptosporidium infections is a significant but reversible phenomenon.23
Systemic Inflammation
One study looking at the effect of systemic inflammation on intestinal barrier function showed that intestinal permeability increased during experimental endotoxemia in humans using E coli lipopolysaccharide at 2 ng/kg. Endotoxemia induced an inflammatory response in subjects, and urinary polyethylene glycol recovery increased during the study, suggesting intestinal permeability is most likely caused by an inflammation-induced increase in intestinal permeability.24
Trauma and Burns
There's evidence that under certain conditions intestinal barrier function may be lost in trauma victims. One study performed on 15 hemodynamically stable burn patients with burns on greater than 20% of their body surfaces showed that those with moderate to major burns had increased intestinal permeability as evidenced by significantly higher lactulose absorption compared with controls.25
Stress
Experiments using animal models have demonstrated that various types of psychological and physical stress can lead to dysfunction of the intestinal barrier via corticotropin-releasing hormone-mediated mast cell activism.26
One human study measured small intestinal permeability via a two-hour lactulose-mannitol urinary excretion test in 23 healthy volunteers who were divided into four groups: control; those receiving indomethacin, a prescription NSAID; those asked to make a public speech; and those anticipating electroshock treatment. Only indomethacin and having to make a public speech, but not anticipation of electroshocks, increased intestinal permeability. Subgroup analysis showed that increased permeability in the public speech group was present only in subjects with significantly elevated levels of cortisol.
In a second study, 13 subjects were pretreated with disodium cromogylcate, a mast cell stabilizer, which prevented increases in the lactulose-mannitol ratio, suggesting acute psychological stress and mast cells may be involved in increased intestinal permeability.27
Medical Conditions Associated With Leaky Gut Syndrome
An increasing number of diseases are recognized as involving alterations in intestinal permeability including autoimmune diseases (eg, type 1 diabetes, celiac disease, multiple sclerosis, rheumatoid arthritis, and Crohn's disease), asthma, IBS, and cancer.13,28 This section examines those conditions that are more readily accepted by the medical establishment to be associated with leaky gut syndrome, as well as those that need further research.
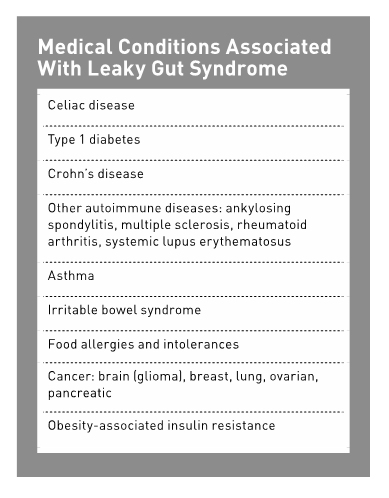
Celiac Disease
Celiac disease is a chronic immune-mediated disorder that occurs in genetically susceptible persons upon ingestion of gluten, a key protein in wheat, barley, and rye, resulting in inflammation of the small bowel mucosa and atrophy of the villi. Symptoms are numerous but may include the classic symptoms of nutrient malabsorption, wasting, and diarrhea. Current prevalence of celiac disease in Western Europeans and in the United States ranges between one in 250 to one in 133.29
There's evidence that gluten itself, via its main protein gliadin, may be directly toxic to the lining of the intestine, thus causing or contributing to increased permeability.2 One ex vivo study, in which human small intestines and intestinal cell monolayers were exposed to gliadin, found that gliadin activated a sustained zonulin release in tissues from celiac patients vs a transient zonulin release in tissues from nonceliac patients. Indeed, biopsies from celiac and nonceliac patients have demonstrated an increase in intestinal permeability, although the level in the nonceliac patients' tissues never reached the level of permeability in the celiac patients' tissues.30
From a functional viewpoint, intestinal permeability hasn't been well assessed in relatives of patients with celiac disease; however, in one study, about one-third of first-degree relatives of celiac patients displayed abnormal permeability, as evidenced by elevated lactulose-mannitol ratios. In addition, the prevalence of celiac disease was much higher in the relatives who were tested and exhibited symptoms of celiac disease.31 In another study of 18 patients with dermatitis herpetiformis, a skin manifestation of celiac disease, who exhibited a wide range of bowel pathology from frank celiac disease to normal intestinal biopsy, all patients had increased intestinal permeability along with elevated serum zonulin levels, including those with no evidence of intestinal disease.32
Type 1 Diabetes
Alterations in intestinal tight junction permeability have been shown to be one of the preceding pathophysiological changes associated with the onset of type 1 diabetes.33 As mentioned, one human study detected elevated zonulin levels in 70% of patients who proceeded to develop type 1 diabetes 3.5 +/- 0.9 years later.15
Another study of diabetic-prone and diabetic-resistant rats showed a fourfold age-related increase in intraluminal zonulin in diabetic-prone rats after 40 days old, detectable only in the small intestine, and was correlated with an increase in intestinal permeability, as well as progression toward full-blown diabetes, suggesting that zonulin may be responsible for early permeability changes and pathogenesis of type 1 diabetes.34
Crohn's Disease
Tumor necrosis factor (TNF)-alpha, a central regulator of inflammation, is intimately related with Crohn's disease, a chronic inflammatory condition of the gastrointestinal tract, and research has shown that TNF-alpha increases paracellular permeability via an effect on tight junctions.35,36 Intestinal permeability in patients with active Crohn's disease normalizes following anti-TNF-alpha therapy with infliximab, a medication for the treatment of certain autoimmune diseases, although it remains unknown whether this healing of the injured mucosa occurs due to the drug therapy or to other independent mechanisims.37,38
Interestingly, increased intestinal permeability has been observed in the absence of Crohn's disease symptoms. There's a case report of a young woman with abnormal permeability who later developed Crohn's disease, suggesting it isn't merely an early manifestation of the disease, but rather is an early step in the pathogenesis of Crohn's.39
Other Autoimmune Diseases
Rheumatologic conditions have long been associated with intestinal function abnormalities, with perhaps the best evidence for this coming from patients with ankylosing spondylitis (AS), a type of arthritis affecting the joints in the spine.40 Increased intestinal permeability has been recognized in these patients, including one study done on 60 patients with AS and 24 of their first-degree relatives, who both had significantly increased small intestinal, but not gastric, permeability compared with controls that could not be explained by NSAID use.41
In addition, multiple sclerosis patients have been observed to have an increased risk of coacquisition of Crohn's disease and both present an increased number of B cells exhibiting CD45RO, a marker of antigen exposure, which supports the idea of preexisting, genetically determined small-intestinal permeability abnormalities with subsequent altered antigen exposure as a pathogenic factor common to both diseases.13
Other autoimmune diseases that have been associated with zonulin as a biomarker include rheumatoid arthritis and systemic lupus erythematosus.42
Asthma
Studies on both adults and children have suggested that intestinal permeability is increased in patients with bronchial asthma. One study of 32 asthmatic children and 32 controls showed significantly higher rates of lactulose excretion in urine and a significantly greater lactulose-mannitol ratio in children with asthma compared with controls. Intestinal permeability didn't correlate with eczema, inhaled steroids, positive skin prick tests to aeroallergens, or severity of asthma.43
Another study looked at intestinal permeability in a group of 37 patients with asthma (21 allergic and 16 nonallergic) by measuring 51-Cr-EDTA in the urine and compared the results to 13 nonasthmatic patients with COPD and 26 healthy controls. Urinary recovery of 51-Cr-EDTA was significantly higher in patients with asthma compared with patients with COPD and controls; however, there was no significant difference in intestinal permeability between patients with allergic vs nonallergic asthma. Also, similar to the findings in the study on asthmatic children, intestinal permeability did not correlate to severity of asthma.44
IBS
IBS is a common functional disorder of the gastrointestinal system, affecting roughly 11% of the population globally. Symptoms may vary among individuals, with common symptoms including abdominal distention, frequent stools, bloating, loose stools, and/or constipation.45
In the pediatric population, there's a recognized association between symptoms of IBS and food hypersensitivity, and, in some cases, true food allergy.46 In a small study of 17 children measuring pre- and postprovocation with suspect foods, it was demonstrated that patients with clinical IBS symptoms developed abnormal permeability, as measured by a change in urinary elimination of lactulose and mannitol after ingestion of the suspect foods. Symptoms disappeared following exclusion of the suspect foods either alone or in combination with cromolyn sodium, an anti-inflammatory medication, leading researchers to believe that IBS may be related to food hypersensitivity linked to intestinal permeability.46
In another study, this time with adults, 54 diarrhea-predominant IBS (D-IBS) patients and 22 controls were evaluated for intestinal permeability using the lactulose-mannitol method. Each completed the functional bowel disease severity index, which comprises three variables: current pain, diagnosis of chronic abdominal pain, and number of physician visits in the past six months. Thirty-nine percent of the D-IBS patients had elevated lactulose-mannitol ratios (≥0.07) vs none of the controls, indicating increased intestinal membrane permeability. In addition, clinical symptoms were positively correlated with increased membrane permeability.47
Food Allergies and Intolerances
In addition to the pediatric study noted above, additional studies have found incidence of increased intestinal permeability in subjects with food allergies and intolerances. In one study of patients with food intolerance, hyperpermeability was observed in approximately one-half of the study population, and other studies have reported a high prevalence of hyperpermeability in patients with either food allergies or hypersensitivity.48
An investigation of 21 patients with food allergy and 20 with food hypersensitivity on an allergen-free diet were divided into four groups based on the seriousness of their clinical symptoms while they were on the allergy-free diet. Statistically significant lactulose-mannitol ratios were found in subjects with both food allergy and hypersensitivity compared with control patients, and the seriousness of symptoms as correlated to lactulose-mannitol ratios also was statistically significant for both food allergy and food hypersensitive subjects. This led researchers to speculate that mechanisms other than the Immunoglobulin E-mediated process, such as breast-feeding or microbial environment, influence the development of oral tolerance in early infancy.49
Cancer
Researchers have identified the hormone receptor guanylyl cyclase C (GC-C), a tumor suppressor that exists in the intestinal tract, as playing a key role in strengthening the body's intestinal barrier and possibly helping to keep cancer at bay. A preclinical study on mice showed that shutting off GC-C led to a compromised intestinal barrier, thus allowing inflammation to occur and cancer-causing agents to seep into the body, damaging DNA and forming cancer outside the intestine, including the liver, lung, and lymph nodes.50
Zonulin also has been identified as a marker in certain cancers including brain (gliomas), breast, lung, ovarian, and pancreatic cancers.51
Obesity-Associated Insulin Resistance
Recent research has suggested that gut health may play a role in obesity. Studies have found circulating zonulin to be significantly increased in obese vs nonobese subjects, and in those with glucose intolerance. One study of 123 men showed circulating zonulin levels to be increased with greater BMIs, waist-to-hip ratio, fasting insulin, fasting triglycerides, uric acid, and Interleukin 6 (IL-6), and negatively associated with HDL and insulin sensitivity, suggesting a relationship between insulin sensitivity and circulating zonulin that may be mediated through an increase in circulating IL-6.11
Conventional and Alternative Treatments
Physicians and clinicians working with patients with leaky gut syndrome may combine conventional medicine with complementary therapies; however, it's important for both patients with increased intestinal permeability and their clinicians to know that evidence-based treatments for leaky gut syndrome are lacking.52
Perhaps the most important first-line therapy for leaky gut syndrome is to remove the potential underlying roots of the problem (eg, gluten, alcohol, and NSAIDs). If possible, any infections should be treated and patients should follow a diet free of any identified food allergens or intolerances. In addition, some practitioners may recommend probiotics, enzymes, and the amino acid L-glutamine to help restore gut function and normalize intestinal permeability.2 Following is an overview of these supplements.
Glutamine
Glutamine, an amino acid, is one of the more studied compounds for reducing intestinal permeability, as glutamine depletion has been shown to result in increased intestinal permeability.48 Glutamine is a major source of energy for enterocytes, absorptive cells in the lining of the intestinal mucosa, and appears to have a special role in restoring normal small bowel permeability and immune function.
Studies to date using glutamine to reduce intestinal permeability have been mixed, with some showing benefit, and others not. One study of 18 children with active Crohn's disease found no benefit in following a glutamine-enriched polymeric diet.53 In a more recent study, however, male patients with Crohn's disease in remission and abnormal intestinal permeability showed significant improvement in intestinal permeability when given either oral glutamine or whey protein (0.5 g/kg ideal body weight/day for two months).54
As a naturally occurring amino acid, glutamine is thought to be a safe supplement when taken at recommended doses of up to 14 g/day; however, it should be used only under medical supervision. Patients who are hypersensitive to MSG should use caution with glutamine, as should patients with epilepsy and/or taking antiseizure drugs.55
Zinc
Zinc, an essential trace element, has been studied for its potential to improve intestinal permeability. In one study involving 12 patients with Crohn's disease in remission, administration of oral zinc sulfate, 110 mg three times per day for eight weeks, resulted in lower lactulose-mannitol ratios, suggesting decreased intestinal permeability with zinc.56
In another study, 111 Bangladeshi children with acute diarrhea and 190 with persistent diarrhea, received, randomly and blind to the investigators, either an elemental zinc supplement of 5 mg/kg body weight/day or a placebo in multivitamin syrup for two weeks. Lactulose excretion was increased in both persistent and acute diarrhea patients at the start of the study, and all patients had significantly reduced lactulose excretion after two weeks of zinc supplementation, with the most significant changes shown in children with E coli, Shigella sp, and Campylobacter jejuni stool isolates.57
Saccharomyces boulardii
Several mechanisms of action of Saccharomyces boulardii, a nonpathogenic yeast, have been identified and include stabilization of the gastrointestinal barrier as it relates to intestinal permeability, regulation of intestinal microbial homeostasis, interference with the ability of pathogens to colonize and infect the mucosa, modulation of immune responses, and induction of enzymatic activity favoring absorption and nutrition.58 One study of 34 patients with Crohn's disease in remission showed an improvement in intestinal permeability as evidenced by a decrease in lactulose-mannitol ratios after the patients were treated with S boulardii.59
Probiotics
Certain probiotics have been found to affect the intestinal barrier favorably by increasing its tightness and reducing inflammation, and there's experimental evidence that some probiotics, especially bifidobacteria, are capable of preventing gliadin-induced toxicity to the intestinal lining.2
In one double-blind, placebo-controlled study the probiotics lactobacilli (Lactobacillus rhamnosus and L reuteri) were administered for six weeks to 41 children with moderate to severe atopic dermatitis. Following probiotic treatment, the frequency of their gastrointestinal symptoms decreased and their lactulose-mannitol ratios decreased, suggesting probiotic supplementation may stabilize intestinal barrier function and decrease gastrointestinal symptoms. In addition, the children's lactulose-mannitol ratios were positively associated with the severity of their eczema.60
A Potential New Treatment and Future Research
Larazotide acetate, a tight junction regulator, is being investigated as a potential treatment for patients with celiac disease who continue to have persistent symptoms, despite being on a gluten-free diet. A double-blind, placebo controlled human clinical trial showed no significant differences in lactulose-mannitol ratios between larazotide acetate and placebo groups. Gluten-induced symptoms, however, as measured by the gastrointestinal symptom rating scale, were significantly more frequent among participants of the group exposed to gluten alone when compared with the gluten plus larazotide acetate and placebo groups. No adverse effects were noted in any of the participants.61,62 A more recent phase-IIb trial recently ended, with investigators announcing positive results and plans for phase-III clinical trials for the definitive assessment of larazotide acetate's efficacy and safety.63
In addition to the larazotide acetate clinical trial that's already under way, another area of research as it potentially relates to the development of leaky gut syndrome is the preliminary study being done on food additives in our industrial food supply. With increasing rates of autoimmune diseases, many of which have been linked to increased intestinal permeability as noted earlier, along with the growing use of food additives in our food supply, research has found that intestinal permeability is increased with not only exposure to gluten and alcohol, but to numerous other food additives as well. These additives include sugars, salt, emulsifiers (eg, mono- and diglycerides), organic solvents, food-grade nanoparticles, and microbial transglutaminase, an enzyme used in a wide variety of food applications including baked goods, meats, and dairy products. While the research is still in its infancy, further studies in this area should enhance researchers' knowledge of the potential role food additives might play in the development of leaky gut syndrome and autoimmune disease.64
Putting It Into Practice
An important takeaway from the research concerning leaky gut syndrome is that although much remains to be learned, including what dietary and pharmaceutical treatments may help normalize intestinal permeability, it's an area of medicine that's increasingly being recognized not only by alternative health practitioners but also by conventional physicians and clinicians. Dietitians, especially those who work closely with patients with celiac disease, Crohn's, and other autoimmune diseases, along with IBS, food allergies and intolerances, asthma, and cancer, are encouraged to stay on top of the ongoing research.
In the meantime, there are several practical strategies dietitians may want to consider for their patients who have been diagnosed with leaky gut syndrome or who are suspected to have the condition, including the following:
• Encourage a diet based mostly on a whole foods, anti-inflammatory eating pattern, such as the Mediterranean diet, with minimal food additives.
• Monitor patients for food allergies and food intolerances and treat them with the appropriate diet as needed.
• Counsel patients with celiac disease on how to manage a strict gluten-free diet, including educating them on avoiding cross-contamination and inadvertent exposure to gluten.
• Consider a gluten-free diet trial in all patients who remain symptomatic despite following the above strategies. It's extremely important, however, to let patients know that a gluten-free diet shouldn't be undertaken unless their physician has first ruled out celiac disease.
• Review alcohol intake and medication use, both prescription and over the counter, with patients. If the patient consumes alcohol, suggest minimal to no intake. If NSAIDs, either prescription or over the counter, are regularly taken, suggest that patients speak with their physicians about possible alternate medications that don't increase intestinal permeability.
• Supplements such as L-glutamine, zinc, probiotics, and S boulardii may be recommended if there are no contraindications per the physician.
• Dietitians also may recommend their patients practice stress management techniques, as acute stress has been shown to lead to increased intestinal permeability.
— E.A. Stewart, MBA, RD, is a dietitian in private practice in southern California specializing in irritable bowel syndrome, celiac disease, and nonceliac gluten sensitivity.
Leaky Gut Syndrome: Putting It Into Practice
Although much remains to be learned about leaky gut syndrome, it is an area of medicine that is increasingly being recognized not only by alternative health practitioners but also by conventional physicians and clinicians.
Dietitians, especially those who work closely with patients with celiac disease, Crohn's and other autoimmune diseases, irritable bowel syndrome, food allergies and intolerances, asthma, and cancer, are encouraged to stay on top of the ongoing research on leaky gut syndrome.
In the meantime, there are several practical nutritional strategies dietitians may want to consider for their patients who have been diagnosed with leaky gut syndrome, or are suspected to have it, including the following:
• Encourage a mostly whole-foods-based, anti-inflammatory diet such as the Mediterranean diet, with minimal food additives.
• Monitor for food allergies or food intolerances and treat with the appropriate diet as needed.
• Counsel patients with celiac disease on how to manage a strict gluten-free diet, including education on avoiding cross-contamination and inadvertent exposure to gluten.
• Consider a gluten-free diet trial in all patients who remain symptomatic despite following the above strategies. It is extremely important, however, to let patients know that a gluten-free diet should not be undertaken unless their physicians have first ruled out celiac disease.
• Review alcohol intake and medications (both prescription and over the counter) with patients. If alcohol is consumed, suggest minimal intake. If NSAIDs, either prescription or over the counter, are regularly taken, suggest patients speak with their physicians about possible alternate medications that do not increase intestinal permeability.
• Supplements, such as L-glutamine, zinc, probiotics, and Saccharomyces boulardii may be recommended if there are no contraindications per the patients' physicians.
• Dietitians may also recommend their patients practice stress management techniques, as acute stress has been shown to lead to increased intestinal permeability.
Learning Objectives
After completing this continuing education course, nutrition professionals should be better able to:
1. Define and discuss leaky gut syndrome.
2. Assess five medical conditions that may be associated with leaky gut syndrome.
3. Distinguish at least three potential causes of leaky gut syndrome.
4. Evaluate at least two treatments that are being researched to help alleviate leaky gut syndrome.
5. Counsel patients on a variety of nutrition strategies that could help normalize intestinal permeability.
CPE Monthly Examination
1. Which of the following proteins is known to regulate intestinal permeability reversibly by modulating intercellular tight junctions?
a. Gluten
b. Gliadin
c. Zonulin
d. Glutenin
2. Which of these autoimmune conditions has the most evidence supporting an association with increased intestinal permeability?
a. Sjögren's syndrome
b. Rheumatoid arthritis
c. Systemic lupus erythematosus
d. Ankylosing spondylitis
3. Which of these nonautoimmune conditions also has been associated with leaky gut syndrome?
a. Skin cancer
b. Asthma
c. Uterine cancer
d. Hypertension
4. Provided there are no contraindications, which of the following supplements may be of benefit to a patient with leaky gut syndrome?
a. Saccharomyces boulardii
b. Vitamin B12
c. Vitamin D
d. Fish oil
5. Leaky gut syndrome may be suspected if which of the following test conditions occurs?
a. Mannitol-lactulose absorption in urine is increased
b. Mannitol-lactulose absorption in urine is decreased
c. Lactulose-mannitol absorption in urine is increased
d. Lactulose-mannitol absorption in urine is decreased
6. What percentage of people with diabetes were found to have elevated zonulin levels in one study referenced in this course?
a. 30%
b. 50%
c. 60%
d. 70%
7. L-glutamine, an amino acid that has been shown to reduce intestinal permeability in some studies, is thought to be safe at what dosage?
a. 14 g/day
b. 18 g/day
c. 20 g/day
d. 25 g/day
8. Under what condition may a gluten-free diet be recommended in patients suspected to have leaky gut syndrome?
a. All patients suspected to have leaky gut syndrome may want to try a gluten-free diet.
b. Patients who do not improve on a traditional anti-inflammatory diet and who have first been tested for celiac disease may want to try a gluten-free diet.
c. Patients who do not respond to therapeutic doses of glutamine may want to try a gluten-free diet.
d. Patients who do not respond to probiotic therapy may want try a gluten-free diet.
9. Which of the following therapies to regulate tight junctions is currently under investigation as a potential treatment for celiac disease patients who continue to have persistent symptoms despite being on a gluten-free diet?
a. Larazotide acetate
b. Zinc
c. Lactobacillus rhamnosus
d. Lactobacillus reuteri
10. Dietitians working with patients with leaky gut syndrome may recommend that their patients reduce or eliminate intake of which of the following drinks?
a. Coffee
b. Tea
c. Wine
d. Milk
References
1. Green P, Jones R. Celiac Disease: A Hidden Epidemic. New York, NY: HarperCollins; 2006:98.
2. Dennis M, Leffler D. Real Life With Celiac Disease: Troubleshooting and Thriving Gluten Free. Bethesda, MD: AGA Press; 2010:228,283,291.
3. Thompson T. The truth about leaky gut. Gluten Free Dietitian website. http://www.glutenfreedietitian.com/dietcom-blog-the-truth-about-leaky-gut/. Updated October 27, 2008. Accessed November 18, 2014.
4. Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11(9):1075-1083.
5. Drake D. New research shows poorly understood "leaky gut syndrome" is real, may be the cause of several diseases. The Daily Beast. http://www.thedailybeast.com/articles/2014/03/27/new-research-shows-poorly-understood-leaky-gut-syndrome-is-real-may-be-the-cause-of-several-diseases.html. Updated March 27, 2014. Accessed March 30, 2015.
6. Jacob A. Gut health and autoimmune disease. Today's Dietitian. 2013;15(2):38-42.
7. Mullin GE. Integrative Gastroenterology. New York, NY: Oxford University Press; 2011:58-59.
8. Menzies IS, Zuckerman MJ, Nukajam WS, et al. Geography of intestinal permeability and absorption. Gut. 1999;44(4):483-489.
9. Nathavitharana KA, Lloyd DR, Raafat F, Brown GA, McNeish AS. Urinary mannitol:lactulose excretion ratios and jejunal mucosal structure. Arch Dis Child. 1988;63:1054-1059.
10. Philpott H, Nandurkar S, Lubel J, Gibson PR. Alternative investigations for irritable bowel syndrome. J Gastroenterol Hepatol. 2013;28(1):73-77.
11. Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7(5):e37160.
12. Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106(39):16799-16804.
13. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91(1):151-175.
14. Researchers find increased zonulin levels among celiac disease patients. University of Maryland Medical Center website. http://umm.edu/news-and-events/news-releases/2000/researchers-find-increased-zonulin-levels-among-celiac-disease-patients. Updated May 8, 2013. Accessed November 18, 2014.
15. Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5)1443-1449.
16. Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42(5):349-361.
17. Bjarnason I, Ward K, Peters TJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet.1984;1:179-182.
18. Nejdfors P, Ekelund M, Weström BR, et al. Intestinal permeability in humans is increased after radiation therapy. Dis Colon Rectum. 2000;43(11):1582-1587.
19. Melichar B, Kohout P, Brátová, M, Solichová D, Králícková P, Zadák Z. Intestinal permeability in patients with chemotherapy-induced stomatitis. J Cancer Res Oncol. 2001;127:314-318.
20. Sigthorsson G, Tibble J, Hayllar J, et al. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43(4):506-511.
21. Jenkins RT, Rooney PJ, Jones DB, Bienenstock J, Goodacre RL. Increased intestinal permeability in patients with rheumatoid arthritis: a side-effect of oral nonsteroidal anti-inflammatory drug therapy? Br J Rheumatol. 1987;26(2):103-107.
22. Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(Pt 24):4435-4440.
23. Zhang Y, Lee B, Thompson M, et al. Lactulose-mannitol intestinal permeability test in children with diarrhea caused by rotavirus and cryptosporidium. Diarrhea Working Group, Peru. J Pediatr Gastroenterol Nutr. 2000;31(1):16-21.
24. Hietbrink F, Besselink MG, Renooij W, et al. Systemic inflammation increases intestinal permeability during experimental human endotoxemia. Shock. 2009;32(4):374-378.
25. Deitch, EA. Intestinal permeability is increased in burn patients shortly after injury. Br J Surg. 1990;77(5):587-592.
26. Söderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001;280(1):G7-G13.
27. Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293-1299.
28. Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn's disease pathogenesis. Ann NY Acad Sci. 2012;1258:159-165.
29. Wakim-Felming J. Celiac disease and malabsorptive disorders. Cleveland Clinic website. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/gastroenterology/celiac-disease-malabsorptive-disorders/Default.htm. Accessed March 30, 2015.
30. Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroentero. 2006;41(4):408-419.
31. Vogelsang H, Wyatt J, Penner E, Lochs H. Screening for celiac disease in first-degree relatives of patients with celiac disease by lactulose/mannitol test. Am J Gastroenterol. 1995:90(10):1838-1842.
32. Smecuol E, Sugai E, Niveloni S, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005;3(4):335-341.
33. Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in celiac disease. Lancet. 2000;355(9214):1518-1519.
34. Fasano A. Intestinal zonulin: open sesame! Gut. 2001;49:159-162.
35. Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16(24)3152-3167.
36. Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171(11):6164-6172.
37. Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97(8):2000-2004.
38. Gibson PR. Increased gut permeability in Crohn's disease: is TNF the link? Gut. 2004;53(12)1724-1725.
39. Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology. 2000;119(6):1740-1744.
40. Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55(10):1512-1520.
41. Vaile JH, Meddings JB, Yacyshyn BR, Russell AS, Maksymowych WP. Bowel permeability and CD45RO expression on circulating CD20+ B cells in patients with ankylosing spondylitis and their relatives. J Rheumatol. 1999;26(1):128-135.
42. Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):71-78.
43. Hijazi Z, Molla AM, Al-Habashi H, Muawad WM, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89(3):227-229.
44. Benard A, Desreumeaux P, Huglo D, Hoorelbeke A, Tonnel AB, Wallaert B. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996;97(6):1173-1178.
45. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
46. Barau E, Dupont C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 1990;11(1):72-77.
47. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1-2):41-46.
48. Rapin JR, Wiernsperger N. Possible links between intestinal permeability and food processing: a potential therapeutic niche for glutamine. Clinics. 2010; 65(6):635-643.
49. Ventura MT, Polimeno L, Amoruso AC, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38(10):732-736.
50. Stronger intestinal barrier may prevent cancer in the rest of the body, new study suggests. Thomas Jefferson University website. http://www.jefferson.edu/web_options/tags/news_fullstory.cfm?articleID=351. Updated March 23, 2012. Accessed December 3, 2014.
51. Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastoenterol H. 2012;10(10):1096-1100.
52. Leaky gut syndrome: what is it? What you should know if you think you have leaky gut syndrome. Web MD website. http://www.webmd.com/digestive-disorders/features/leaky-gut-syndrome. Updated August 14, 2013. Accessed December 3, 2014.
53. Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double-blinded randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn's disease. J Pediatr Gastroenterol. 2000;30(1):78-84.
54. Benjamin J, Makharia G, Ahuja V, et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn's disease: a randomized controlled trial. Digest Dis Sci. 2012;57(4):1000-1012.
55. Glutamine. Beth Israel Deaconess Medical Center website. http://www.bidmc.org/YourHealth/Holistic-Health/Herbs-and-Supplements.aspx?ChunkID=21749#P4. Updated September 2014. Accessed December 3, 2014.
56. Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Incà R. Zinc supplementation tightens "leaky gut" in Crohn's disease. Inflamm Bowel Dis. 2001;7(2):94-98.
57. Roy SK, Behrens RH, Haider R, et al. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenteral Nutr. 1992;15(3):289-296.
58. Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5(2):111-125.
59. Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, et al. Influence of saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol. 2008;43(7):842-848.
60. Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr. 2004;145(5):612-616.
61. Kelly CP, Green PH, Murray JA, et al. Larazotide acetate in patients with celiac disease undergoing a gluten challenge: a randomized placebo-controlled study. Aliment Pharmacol Ther. 2013;37(2):252-262.
62. Fasano A, Flaherty S. Gluten Freedom. New York, NY: Turner Publishing Company; 2014:273.
63. Alba Therapeutics announces positive results of phase IIb trial in celiac disease. PR Newswire. February 11, 2014. http://www.prnewswire.com/news-releases/alba-therapeutics-announces-positive-results-of-phase-iib-trial-in-celiac-disease-244877451.html. Accessed December 3, 2014.
64. Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14(6):479-489.
